Insulin glargine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Insulin glargine is a recombinant insulin analog that is FDA approved for the {{{indicationType}}} of type 1 diabetes mellitus and type 2 diabetes mellitus.. Common adverse reactions include hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, edema and weight gain..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Lantus is indicated to improve glycemic control in adults and pediatric patients with type 1 diabetes mellitus and in adults with type 2 diabetes mellitus.
- important limitations of use:
- Lantus is not recommended for the treatment of diabetic ketoacidosis. intravenous short-acting insulin is the preferred treatment for this condition.
- LANTUS is a recombinant human insulin analog for once daily subcutaneous administration with potency that is approximately the same as the potency of human insulin. LANTUS exhibits a relatively constant glucose-lowering profile over 24 hours that permits once-daily dosing.
- LANTUS may be administered at any time during the day. LANTUS should be administered subcutaneously once a day at the same time every day. The dose of LANTUS must be individualized based on clinical response. Blood glucose monitoring is essential in all patients receiving insulin therapy.
- Patients adjusting the amount or timing of dosing with LANTUS, should only do so under medical supervision with appropriate glucose monitoring [see Warnings and Precautions (5.1).]
- In patients with type 1 diabetes, LANTUS must be used in regimens with short-acting insulin.
- The intended duration of activity of LANTUS is dependent on injection into subcutaneous tissue [see Clinical pharmacology (12.2)]. LANTUS should not be administered intravenously or via an insulin pump. Intravenous administration of the usual subcutaneous dose could result in severe hypoglycemia [see Warnings and Precautions (5.3)].
- As with all insulins, injection sites should be rotated within the same region (abdomen, thigh, or deltoid) from one injection to the next to reduce the risk of lipodystrophy [See Adverse Reactions (6.1)].
- In clinical studies, there was no clinically relevant difference in insulin glargine absorption after abdominal, deltoid, or thigh subcutaneous administration. As for all insulins, the rate of absorption, and consequently the onset and duration of action, may be affected by exercise and other variables, such as stress, intercurrent illness, or changes in co-administered drugs or meal patterns.
- Initiation of LANTUS therapy
- The recommended starting dose of LANTUS in patients with type 1 diabetes should be approximately one-third of the total daily insulin requirements. Short-acting, premeal insulin should be used to satisfy the remainder of the daily insulin requirements.
- The recommended starting dose of LANTUS in patients with type 2 diabetes who are not currently treated with insulin is 10 units (or 0.2 Units/kg) once daily, which should subsequently be adjusted to the patient's needs.
- The dose of LANTUS should be adjusted according to blood glucose measurements. The dosage of LANTUS should be individualized under the supervision of a healthcare provider in accordance with the needs of the patient.
- Converting to LANTUS from other insulin therapies
- If changing from a treatment regimen with an intermediate-or long-acting insulin to a regimen with LANTUS, the amount and timing of shorter-acting insulins and doses of any oral anti-diabetic drugs may need to be adjusted.
- If transferring patients from once-daily NPH insulin to once-daily LANTUS, the recommended initial LANTUS dose is the same as the dose of NPH that is being discontinued.
- If transferring patients from twice-daily NPH insulin to once-daily LANTUS, the recommended initial LANTUS dose is 80% of the total NPH dose that is being discontinued. This dose reduction will lower the likelihood of hypoglycemia [see Warnings and Precautions (5.3)].
- Dosage Forms And Strengths
- LANTUS solution for injection 100 Units per mL is available as:
- 10 mL Vial (1000 Units/10 mL)
- 3 mL SoloStar® disposable insulin device (300 Units/3 mL)
- LANTUS solution for injection 100 Units per mL is available as:
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Insulin glargine in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Insulin glargine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Insulin glargine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Insulin glargine in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Insulin glargine in pediatric patients.
Contraindications
- Condition1
Warnings
- Description
Precautions
- Description
- Hypoglycemia
- Hypoglycemia is the most common adverse reaction of insulin, including LANTUS. The risk of hypoglycemia increases with intensive glycemic control. Patients must be educated to recognize and manage hypoglycemia. Severe hypoglycemia can lead to unconsciousness or convulsions and may result in temporary or permanent impairment of brain function or death. Severe hypoglycemia requiring the assistance of another person or parenteral glucose infusion or glucagon administration has been observed in clinical trials with insulin, including trials with LANTUS.
- The timing of hypoglycemia usually reflects the time-action profile of the administered insulin formulations. Other factors such as changes in food intake (e.g., amount of food or timing of meals), exercise, and concomitant medications may also alter the risk of hypoglycemia [See Drug Interactions (7)].
- The prolonged effect of subcutaneous LANTUS may delay recovery from hypoglycemia. Patients being switched from twice daily NPH insulin to once-daily LANTUS should have their initial LANTUS dose reduced by 20% from the previous total daily NPH dose to reduce the risk of hypoglycemia.
- As with all insulins, use caution in patients with hypoglycemia unawareness and in patients who may be predisposed to hypoglycemia (e.g., the pediatric population and patients who fast or have erratic food intake). The patient's ability to concentrate and react may be impaired as a result of hypoglycemia. This may present a risk in situations where these abilities are especially important, such as driving or operating other machinery.
- Early warning symptoms of hypoglycemia may be different or less pronounced under certain conditions, such as longstanding diabetes, diabetic neuropathy, use of medications such as beta-blockers, or intensified glycemic control. These situations may result in severe hypoglycemia (and, possibly, loss of consciousness) prior to the patient's awareness of hypoglycemia.
- Hypersensitivity and allergic reactions
- Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products, including LANTUS.
Adverse Reactions
Clinical Trials Experience
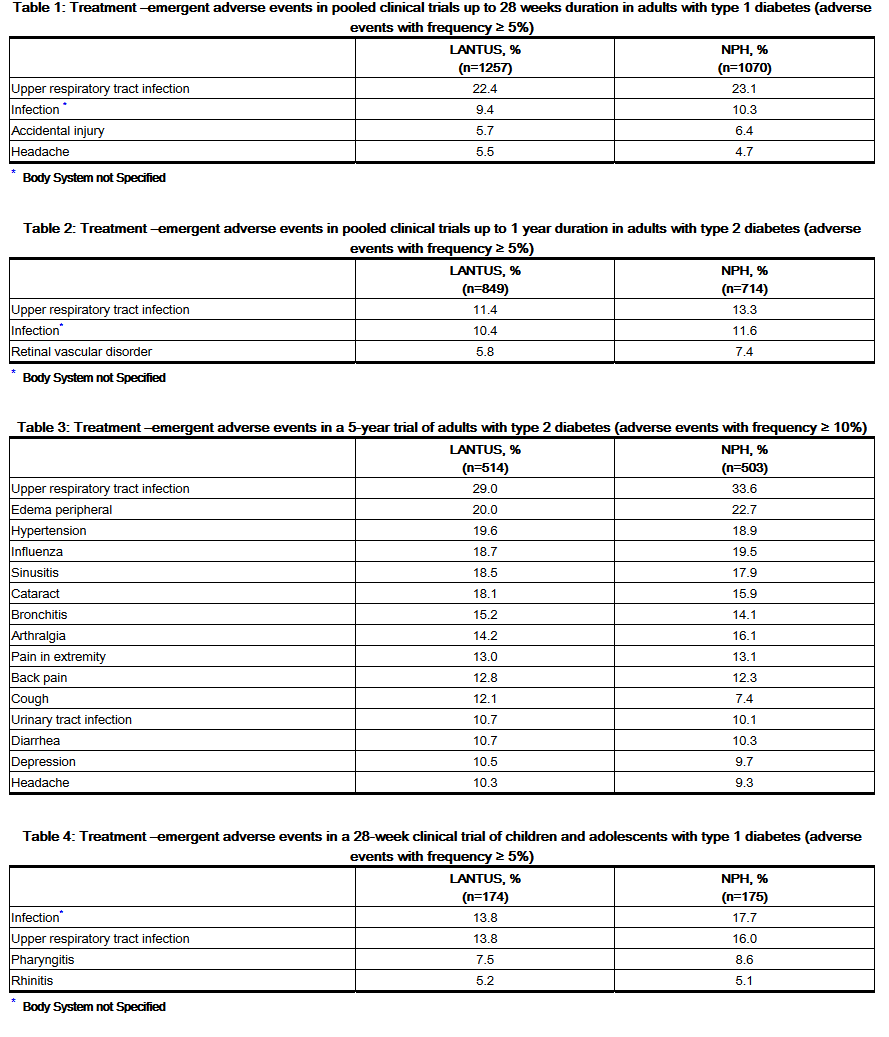
- Because clinical trials are conducted under widely varying designs, the adverse reaction rates reported in one clinical trial may not be easily compared to those rates reported in another clinical trial, and may not reflect the rates actually observed in clinical practice.
- The frequencies of treatment-emergent adverse events during LANTUS clinical trials in patients with type 1 diabetes mellitus and type 2 diabetes mellitus are listed in the tables below.
- Severe Hypoglycemia
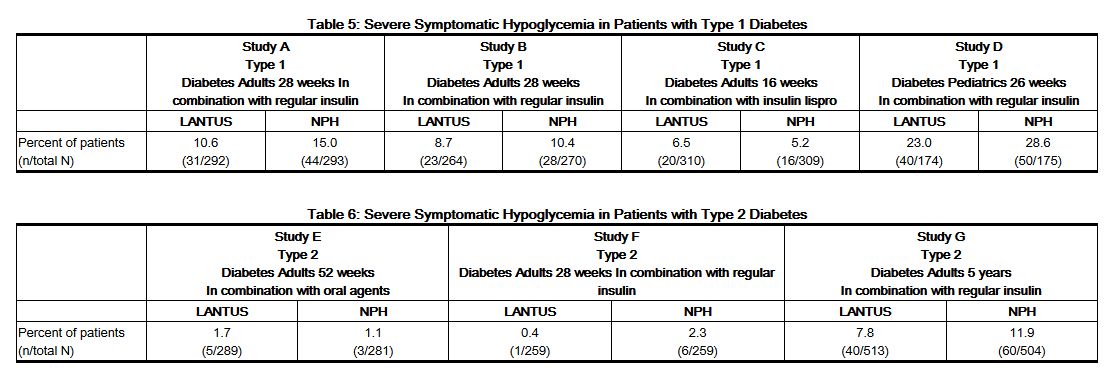
- Hypoglycemia is the most commonly observed adverse reaction in patients using insulin, including LANTUS [See Warnings and Precautions (5.3)]. Tables 5, and 6 and 7 summarize the incidence of severe hypoglycemia in the LANTUS individual clinical trials. Severe symptomatic hypoglycemia was defined as an event with symptoms consistent with hypoglycemia requiring the assistance of another person and associated with either a blood glucose below 50 mg/dL (≤56 mg/dL in the 5-year trial and ≤36 mg/dL in the ORIGIN trial) or prompt recovery after oral carbohydrate, intravenous glucose or glucagon administration.
- The proportion of patients experiencing severe symptomatic hypoglycemia in the LANTUS clinical trials [See Clinical Studies (14)] in adults with type 1 diabetes and type 2 diabetes were comparable for all treatment regimens (see Tables 5 and 6). In the pediatric phase 3 clinical trial, children and adolescents with type 1 diabetes had a higher incidence of severe symptomatic hypoglycemia in the two treatment groups compared to the adult trials with type 1 diabetes.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Insulin glargine in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Insulin glargine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Insulin glargine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Insulin glargine with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Insulin glargine with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Insulin glargine with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Insulin glargine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Insulin glargine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Insulin glargine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Insulin glargine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Insulin glargine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Insulin glargine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Insulin glargine in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Insulin glargine in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Insulin glargine in the drug label.
Pharmacology
There is limited information regarding Insulin glargine Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Insulin glargine in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Insulin glargine in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Insulin glargine in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Insulin glargine in the drug label.
How Supplied
Storage
There is limited information regarding Insulin glargine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Insulin glargine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Insulin glargine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Insulin glargine in the drug label.
Precautions with Alcohol
- Alcohol-Insulin glargine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Insulin glargine |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Insulin glargine |Label Name=Insulin glargine11.png
}}
{{#subobject:
|Label Page=Insulin glargine |Label Name=Insulin glargine11.png
}}