Colesevelam: Difference between revisions
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
No edit summary |
||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{ | {{Colesevelam}} | ||
{{CMG}}; {{AE}} {{SS}} | |||
}} | |||
{{CMG}} | |||
'''''For patient information about Colesevelam, click [[Colesevelam (patient information)|here]].''''' | |||
{{SB}} WELCHOL<sup>®</sup> | |||
==[[Colesevelam | ==Overview== | ||
'''Colesevelam''' is a [[bile acid sequestrant]] administered orally. It is developed by [[Genzyme]] and marketed in the US by Daiichi Sankyo under the brand name '''Welchol''' and elsewhere by Genzyme as '''Cholestagel'''. In Canada it is marketed by Valeant as '''Lodalis'''. | |||
==Category== | |||
Cardiovasuclar Drugs:Bile acid sequestrants | |||
==FDA Package Insert== | |||
''' [[Colesevelam indications and usage|Indications and Usage]]''' | |||
'''| [[Colesevelam dosage and administration|Dosage and Administration]]''' | |||
'''| [[Colesevelam dosage forms and strengths|Dosage Forms and Strengths]]''' | |||
'''| [[Colesevelam contraindications|Contraindications]]''' | |||
'''| [[Colesevelam warnings and precautions|Warnings and Precautions]]''' | |||
'''| [[Colesevelam adverse reactions|Adverse Reactions]]''' | |||
'''| [[Colesevelam drug interactions|Drug Interactions]]''' | |||
'''| [[Colesevelam use in specific populations|Use in Specific Populations]]''' | |||
'''| [[Colesevelam overdosage|Overdosage]]''' | |||
'''| [[Colesevelam description|Description]]''' | |||
'''| [[Colesevelam clinical pharmacology|Clinical Pharmacology]]''' | |||
'''| [[Colesevelam nonclinical toxicology|Nonclinical Toxicology]]''' | |||
'''| [[Colesevelam clinical studies|Clinical Studies]]''' | |||
'''| [[Colesevelam how supplied storage and handling|How Supplied/Storage and Handling]]''' | |||
'''| [[Colesevelam patient counseling information|Patient Counseling Information]]''' | |||
'''| [[Colesevelam labels and packages|Labels and Packages]]''' | |||
== | ==Mechanism of Action== | ||
==References== | |||
{{Reflist|2}} | |||
{{Lipid modifying agents}} | {{Lipid modifying agents}} | ||
[[Category:Hepatology]] | |||
[[Category:Bile acid sequestrants]] | |||
[[Category:Cardiovasuclar Drugs]] | |||
[[Category:Drugs]] | [[Category:Drugs]] | ||
Revision as of 17:14, 10 February 2014
 | |
| Clinical data | |
|---|---|
| Trade names | Welchol, Cholestagel |
| MedlinePlus | a699050 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Metabolism | Colesevelam is not absorbed and not metabolised. |
| Elimination half-life | N/A (non-systemic drug) |
| Excretion | By intestines only, colesevelam is non-systemic. |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
For patient information about Colesevelam, click here.
Synonyms / Brand Names: WELCHOL®
Overview
Colesevelam is a bile acid sequestrant administered orally. It is developed by Genzyme and marketed in the US by Daiichi Sankyo under the brand name Welchol and elsewhere by Genzyme as Cholestagel. In Canada it is marketed by Valeant as Lodalis.
Category
Cardiovasuclar Drugs:Bile acid sequestrants
FDA Package Insert
Indications and Usage | Dosage and Administration | Dosage Forms and Strengths | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Use in Specific Populations | Overdosage | Description | Clinical Pharmacology | Nonclinical Toxicology | Clinical Studies | How Supplied/Storage and Handling | Patient Counseling Information | Labels and Packages
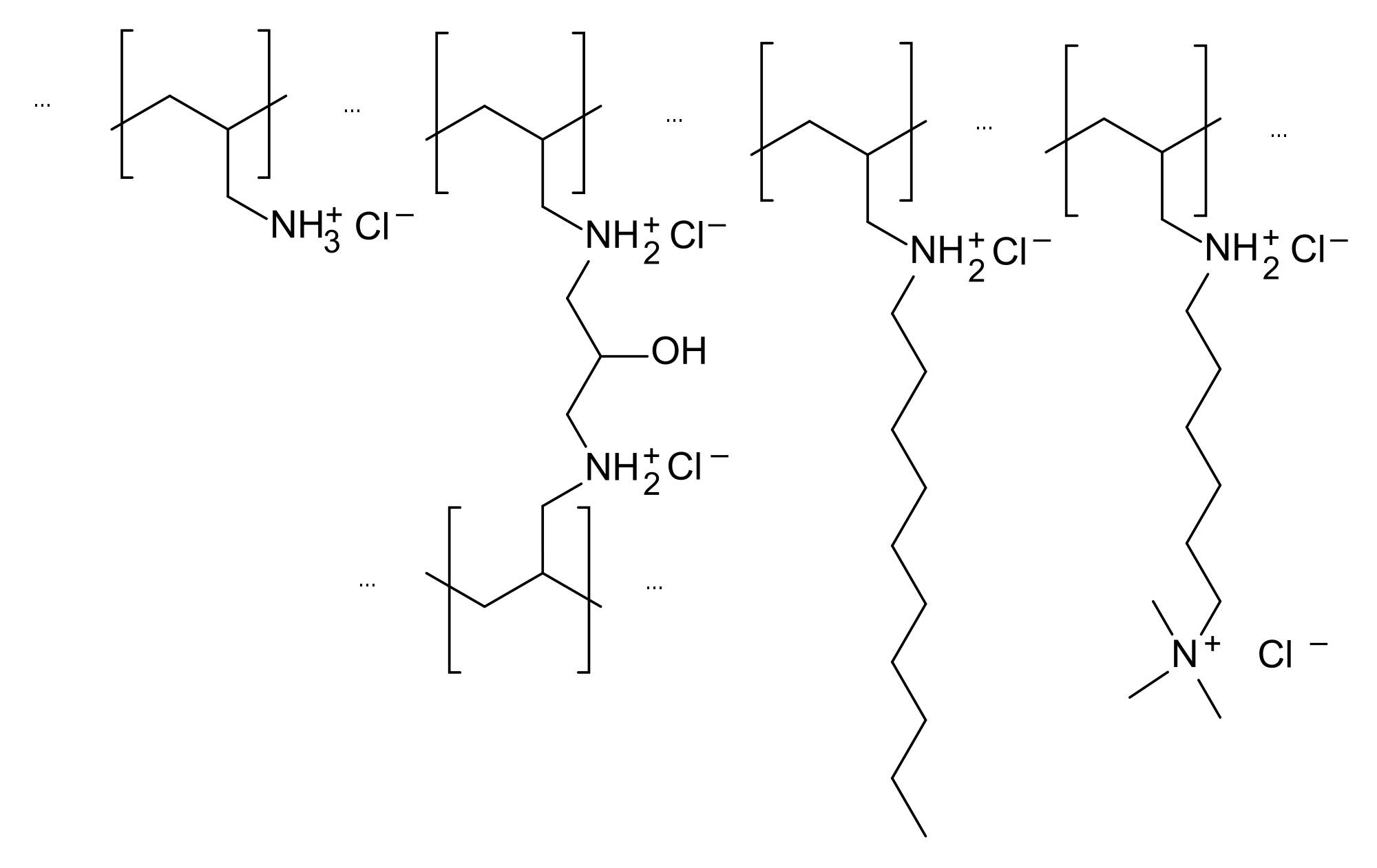
Mechanism of Action
References
- Pages using duplicate arguments in template calls
- Pages with script errors
- Drugs with non-standard legal status
- Articles with changed ChemSpider identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without InChI source
- Drugboxes which contain changes to verified fields
- Infobox drug tracked parameters
- Hepatology
- Bile acid sequestrants
- Cardiovasuclar Drugs
- Drugs