Eplerenone: Difference between revisions
Matt Pijoan (talk | contribs) m (Protected "Eplerenone": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
m (Bot: Automated text replacement (-{{SIB}} + & -{{EJ}} + & -{{EH}} + & -{{Editor Join}} + & -{{Editor Help}} +)) |
||
| Line 20: | Line 20: | ||
{{CMG}} | {{CMG}} | ||
==[[Eplerenone (patient information)|For patient information, click here]]== | ==[[Eplerenone (patient information)|For patient information, click here]]== | ||
Revision as of 02:20, 9 August 2012
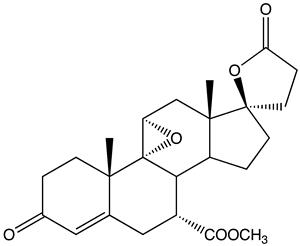 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 69% |
| Metabolism | hepatic (CYP3A4) |
| Elimination half-life | 3–5 hours |
| Excretion | 67% renal 32% biliary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C24H30O6 |
| Molar mass | 414.49 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
For patient information, click here
Eplerenone (INN) (Template:PronEng) is an aldosterone antagonist used as an adjunct in the management of chronic heart failure. It is similar to spironolactone, though it may be more specific for the mineralocorticoid receptor and is specifically marketed for reducing cardiovascular risk in patients following myocardial infarction. It is marketed by Pfizer under the trade name Inspra.
Clinical use
Indications
Eplerenone is specifically indicated for the reduction of risk of cardiovascular death in patients with heart failure and left ventricular dysfunction within 3–14 days of an acute myocardial infarction, in combination with standard therapies and as treatment against hypertension.
Contraindications
Eplerenone is contraindicated in patients with hyperkalaemia, severe renal impairment (creatinine Cl less than 30 ml/min), or severe hepatic impairment (Child-Pugh C). The manufacturer of eplerenone also contraindicates ( relative C.I. ) concomitant treatment with ketoconazole, itraconazole or other potassium-sparing diuretics (though the manufacturer still considers taking these drugs to be absolute C.I.) Potential benefits should be weighted against possible risks.
Adverse effects
Common adverse drug reactions (ADRs) associated with the use of eplerenone include: hyperkalaemia, hypotension, dizziness, altered renal function, and increased creatinine concentration.[1]
Drug interactions
Eplerenone is primarily metabolised by the cytochrome P450 enzyme CYP3A4. Thus the potential exists for adverse drug interactions with other drugs that induce or inhibit CYP3A4. Specifically, the concomitant use of the CYP3A4 potent inhibitors ketoconazole and itraconazole is contraindicated. Other CYP3A4 inhibitors including erythromycin, saquinavir, and verapamil should be used with caution. Other drugs that increase potassium concentrations may increase the risk of hyperkalaemia associated with eplerenone therapy, including salt substitutes,[2] potassium supplements and other potassium-sparing diuretics.
General considerations
Due to the high risk of elevated potassium levels in individuals taking eplerenone, The United States FDA suggests routine checks on the individual's potassium level to screen for hyperkalemia.
See also
References
- ↑ Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006
- ↑ LoSalt Advisory Statement (PDF)
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Aldosterone antagonists
- Drugs