Upper gastrointestinal bleeding pathophysiology: Difference between revisions
Usama Talib (talk | contribs) No edit summary |
Aditya Ganti (talk | contribs) No edit summary |
||
| Line 32: | Line 32: | ||
==Pathophysiology== | ==Pathophysiology== | ||
===Blood supply of Foregut=== | |||
The digestive system is supplied by the celiac artery. The celiac artery is the first major branch from the abdominal aorta, and is the only major artery that nourishes the digestive organs.<ref name="pmid18730308">{{cite journal |vauthors=Feldman SE |title=Blood supply to stomach |journal=Calif Med |volume=112 |issue=4 |pages=55 |year=1970 |pmid=18730308 |pmc=1501289 |doi= |url=}}</ref><ref name="pmid26140727">{{cite journal |vauthors=Granger DN, Holm L, Kvietys P |title=The Gastrointestinal Circulation: Physiology and Pathophysiology |journal=Compr Physiol |volume=5 |issue=3 |pages=1541–83 |year=2015 |pmid=26140727 |doi=10.1002/cphy.c150007 |url=}}</ref><ref name="pmid11355897">{{cite journal |vauthors=Geboes K, Geboes KP, Maleux G |title=Vascular anatomy of the gastrointestinal tract |journal=Best Pract Res Clin Gastroenterol |volume=15 |issue=1 |pages=1–14 |year=2001 |pmid=11355897 |doi=10.1053/bega.2000.0152 |url=}}</ref><ref name="pmid986621">{{cite journal |vauthors=Varga F, Csáky TZ |title=Changes in the blood supply of the gastrointestinal tract in rats with age |journal=Pflugers Arch. |volume=364 |issue=2 |pages=129–33 |year=1976 |pmid=986621 |doi= |url=}}</ref><ref name="pmid4599528">{{cite journal |vauthors=Matuchansky C, Bernier JJ |title=[Prostaglandins and the digestive tract] |language=French |journal=Biol Gastroenterol (Paris) |volume=6 |issue=3 |pages=251–68 |year=1973 |pmid=4599528 |doi= |url=}}</ref><ref name="pmid4372738">{{cite journal |vauthors=Radbil' OS |title=[Prostaglandins and the digestive system organs] |language=Russian |journal=Ter. Arkh. |volume=46 |issue=4 |pages=6–14 |year=1974 |pmid=4372738 |doi= |url=}}</ref><ref name="pmid6990725">{{cite journal |vauthors=Robert A |title=Prostaglandins and digestive diseases |journal=Adv Prostaglandin Thromboxane Res |volume=8 |issue= |pages=1533–41 |year=1980 |pmid=6990725 |doi= |url=}}</ref> | |||
{| class="wikitable" | |||
! colspan="2" |Foregut | |||
!Blood supply | |||
|- | |||
| rowspan="3" |'''<u>Esophagus</u>''' | |||
| | |||
Upper esophageal sphincter<br> | |||
Cervical esophagus | |||
| Inferior thyroid artery | |||
|- | |||
|Thoracic esophagus | |||
|Aortic esophageal arteries or branches of the bronchial arteries | |||
|- | |||
| | |||
Distal esophagus<br> | |||
Lower esophageal sphincter | |||
|Left gastric artery and left phrenic artery | |||
|- | |||
| rowspan="3" |'''<u>Stomach</u>''' | |||
|Lesser curvature | |||
|Right and left gastric arteries | |||
|- | |||
|Greater curvature | |||
|Right and left gastroepiploic arteries | |||
|- | |||
|Gastric fundus | |||
|Short gastric arteries | |||
|- | |||
| rowspan="2" |'''<u>Duodenum</u>''' | |||
|First and second parts | |||
| | |||
Gastroduodenal artery (GDA) and<br> | |||
Superior pancreaticoduodenal artery | |||
|- | |||
|Third and fourth parts | |||
|Inferior pancreaticoduodenal artery | |||
|} | |||
[[Image:Stomach blood supply.svg.png|frame|center|Blood supply of stomach<br> Source: By Mikael Häggström.https://commons.wikimedia.org/w/index.php?curid=3416062]] | |||
===Mucosal barrier=== | |||
*The gastric mucosa is protected from the acidic environment by mucus, bicarbonate, prostaglandins, and blood flow.<ref name="pmid6846549">{{cite journal |vauthors=Hills BA, Butler BD, Lichtenberger LM |title=Gastric mucosal barrier: hydrophobic lining to the lumen of the stomach |journal=Am. J. Physiol. |volume=244 |issue=5 |pages=G561–8 |year=1983 |pmid=6846549 |doi= |url=}}</ref><ref name="pmid2657286">{{cite journal |vauthors=Clamp JR, Ene D |title=The gastric mucosal barrier |journal=Methods Find Exp Clin Pharmacol |volume=11 Suppl 1 |issue= |pages=19–25 |year=1989 |pmid=2657286 |doi= |url=}}</ref><ref name="pmid10677782">{{cite journal |vauthors=Werther JL |title=The gastric mucosal barrier |journal=Mt. Sinai J. Med. |volume=67 |issue=1 |pages=41–53 |year=2000 |pmid=10677782 |doi= |url=}}</ref> | |||
*This mucosal barrier consists of three protective components which include: | |||
**Layer of epithelial cell lining. | |||
**Layer of mucus, secreted by surface epithelial cells and foveolar cells. | |||
**Layer of bicarbonate ions, secreted by the surface epithelial cells. | |||
[[Image: Stomach mucosal layer labeled.svg.png|center|frame|Diagram of alkaline Mucous layer in stomach with mucosal defense mechanisms<br> '''Source''': By M•Komorniczak (http://creativecommons.org/licenses/by/3.0)], via Wikimedia Commons]] | |||
The following table demonstrates the defense mechanisms of gastric mucosal barrier<ref name="pmid3072665">{{cite journal |vauthors=Forssell H |title=Gastric mucosal defence mechanisms: a brief review |journal=Scand. J. Gastroenterol. Suppl. |volume=155 |issue= |pages=23–8 |year=1988 |pmid=3072665 |doi= |url=}}</ref> | |||
{| class="wikitable" | |||
! colspan="2" |Defense mechanisms of gastric mucosal barrier | |||
|- | |||
|Mucus layer | |||
|Forms a protective gel-like coating over the entire gastric mucosal surface | |||
|- | |||
|Epithelial layer | |||
|Epithelial cell layer are bound by tight junctions that repel fluids | |||
|- | |||
|Bicarbonate ions | |||
|Neutralize acids | |||
|} | |||
===Pathogenesis=== | ===Pathogenesis=== | ||
The main inciting event in the pathogeneis of upper GI bleeding is damage to mucosal injury. This mucosal injury can occur at various levels of GI tract. If the damage and bleeding is confined up to ligament of Treitz, it is defined as upper GI bleeding.<ref name="pmid18346679">{{cite journal |vauthors=van Leerdam ME |title=Epidemiology of acute upper gastrointestinal bleeding |journal=Best Pract Res Clin Gastroenterol |volume=22 |issue=2 |pages=209–24 |year=2008 |pmid=18346679 |doi=10.1016/j.bpg.2007.10.011 |url=}}</ref><ref name="pmid15173790">{{cite journal |vauthors=Boonpongmanee S, Fleischer DE, Pezzullo JC, Collier K, Mayoral W, Al-Kawas F, Chutkan R, Lewis JH, Tio TL, Benjamin SB |title=The frequency of peptic ulcer as a cause of upper-GI bleeding is exaggerated |journal=Gastrointest. Endosc. |volume=59 |issue=7 |pages=788–94 |year=2004 |pmid=15173790 |doi= |url=}}</ref> | |||
{| class="wikitable" | |||
* | !Etiology | ||
!Frequency of occurance | |||
* | |- | ||
*The | |Peptic ulcer disease | ||
* | |50% | ||
|- | |||
== | |Variceal bleeding | ||
* | |20% | ||
* | |- | ||
|Esophagitis, gastritis, and duodenitis | |||
== | |10-15% | ||
|- | |||
|Mallory-Weiss tear | |||
|15% | |||
|- | |||
|Malignancy | |||
|3-5% | |||
|- | |||
|Arteriovenous malformation | |||
|<3% | |||
|- | |||
|Gastric antral vascular ectasia | |||
|<1% | |||
|- | |||
|Dieulafoy lesion | |||
|<1% | |||
|} | |||
===Pathogenesis=== | |||
*Regardless of etiology, if the balance of gastric acid secretion and mucosal defenses is disrupted, acid interacts with the epithelium to cause damage.<ref name="pmid6499">{{cite journal |vauthors=Gartner AH |title=Aspirin-induced gastritis and gastrointestinal bleeding |journal=J Am Dent Assoc |volume=93 |issue=1 |pages=111–7 |year=1976 |pmid=6499 |doi= |url=}}</ref><ref name="pmid23555156">{{cite journal |vauthors=Iwamoto J, Saito Y, Honda A, Matsuzaki Y |title=Clinical features of gastroduodenal injury associated with long-term low-dose aspirin therapy |journal=World J. Gastroenterol. |volume=19 |issue=11 |pages=1673–82 |year=2013 |pmid=23555156 |pmc=3607744 |doi=10.3748/wjg.v19.i11.1673 |url=}}</ref><ref name="pmid8898449">{{cite journal |vauthors=Hawkey CJ |title=Non-steroidal anti-inflammatory drug gastropathy: causes and treatment |journal=Scand. J. Gastroenterol. Suppl. |volume=220 |issue= |pages=124–7 |year=1996 |pmid=8898449 |doi= |url=}}</ref> | |||
**Varices are large, tortuous veins and protrude into the lumen, rupturing.<ref name="pmid26467538">{{cite journal |vauthors=Quan S, Yang H, Tanyingoh D, Villeneuve PJ, Stieb DM, Johnson M, Hilsden R, Madsen K, van Zanten SV, Novak K, Lang E, Ghosh S, Kaplan GG |title=Upper gastrointestinal bleeding due to peptic ulcer disease is not associated with air pollution: a case-crossover study |journal=BMC Gastroenterol |volume=15 |issue= |pages=131 |year=2015 |pmid=26467538 |pmc=4604641 |doi=10.1186/s12876-015-0363-6 |url=}}</ref> | |||
**Helicobacter pylori disrupts the mucosal barrier and causes inflammation of the mucosa of the stomach and duodenum.<ref name="Quan2002">{{cite journal|last1=Quan|first1=C|title=Management of peptic ulcer disease not related to Helicobacter pylori or NSAIDs|journal=The American Journal of Gastroenterology|volume=97|issue=12|year=2002|pages=2950–2961|issn=00029270|doi=10.1016/S0002-9270(02)05485-0}}</ref><ref name="MalfertheinerChan2009">{{cite journal|last1=Malfertheiner|first1=Peter|last2=Chan|first2=Francis KL|last3=McColl|first3=Kenneth EL|title=Peptic ulcer disease|journal=The Lancet|volume=374|issue=9699|year=2009|pages=1449–1461|issn=01406736|doi=10.1016/S0140-6736(09)60938-7}}</ref> | |||
**As the ulcer progresses beyond the mucosa to the submucosa the inflammation causes weakening and necrosis of arterial walls, leading to pseudoaneurysm formation followed by rupture and hemorrhage.<ref name="pmid25516672">{{cite journal |vauthors=Quan S, Frolkis A, Milne K, Molodecky N, Yang H, Dixon E, Ball CG, Myers RP, Ghosh S, Hilsden R, van Zanten SV, Kaplan GG |title=Upper-gastrointestinal bleeding secondary to peptic ulcer disease: incidence and outcomes |journal=World J. Gastroenterol. |volume=20 |issue=46 |pages=17568–77 |year=2014 |pmid=25516672 |pmc=4265619 |doi=10.3748/wjg.v20.i46.17568 |url=}}</ref> | |||
**NSAIDs inhibit cyclooxygenase, leading to impaired mucosal defenses by decreasing mucosal prostaglandin synthesis.<ref name="pmid26870237">{{cite journal |vauthors=Xi B, Jia JJ, Lin BY, Geng L, Zheng SS |title=Peptic ulcers accompanied with gastrointestinal bleeding, pylorus obstruction and cholangitis secondary to choledochoduodenal fistula: A case report |journal=Oncol Lett |volume=11 |issue=1 |pages=481–483 |year=2016 |pmid=26870237 |pmc=4727103 |doi=10.3892/ol.2015.3908 |url=}}</ref> | |||
**During stress, there is acid hypersecretion; therefore, the breakdown of mucosal defenses leads to injury of the mucosa and subsequent bleeding. | |||
**Mucosal defects along with dilated and tortuous vessels in dieulafoy lesion put them at risk for rupture because of necrosis of the arterial wall from exposure to gastric acid.<ref name="pmid313784">{{cite journal |vauthors=Stern AI, Korman MG, Hunt PS, Hansky J, Hillman HS, Schmidt GT |title=The Mallory-Weiss lesion as a cause of upper gastrointestinal bleeding |journal=Aust N Z J Surg |volume=49 |issue=1 |pages=13–8 |year=1979 |pmid=313784 |doi= |url=}}</ref><ref name="pmid8307643">{{cite journal |vauthors=Katz PO, Salas L |title=Less frequent causes of upper gastrointestinal bleeding |journal=Gastroenterol. Clin. North Am. |volume=22 |issue=4 |pages=875–89 |year=1993 |pmid=8307643 |doi= |url=}}</ref><ref name="pmid17633871">{{cite journal |vauthors=Sabljak P, Velicković D, Stojakov D, Bjelović M, Ebrahimi K, Spica B, Sljukić V, Pesko P |title=[Less frequent causes of upper gastrointestinal bleeding] |journal=Acta Chir Iugosl |volume=54 |issue=1 |pages=119–23 |year=2007 |pmid=17633871 |doi= |url=}}</ref><ref name="pmid11727185">{{cite journal |vauthors=Depolo A, Dobrila-Dintinjana R, Uravi M, Grbas H, Rubini M |title=[Upper gastrointestinal bleeding - Review of our ten years results] |language=German |journal=Zentralbl Chir |volume=126 |issue=10 |pages=772–6 |year=2001 |pmid=11727185 |doi=10.1055/s-2001-18265 |url=}}</ref> | |||
{{familytree/start}} | |||
{{familytree | | | | | | | | | | A01 | | | | | |A01=NSAIDS}} | |||
{{familytree | | | | | | | | | | |!| | | | | | | | }} | |||
{{familytree | | | | | | | | | | A01 | | | | | |A01=Inhibits cycloxygenase pathway}} | |||
{{familytree | | | | | | | | | | |!| | | | | | | | }} | |||
{{familytree | | | | | |,|-|-|-|-|^|-|-|-|-|-|.| | | }} | |||
{{familytree | | | | | B01 | | | | | | | | | B02 |B01=COX-1|B02=COX-2}} | |||
{{familytree | | | | | |!| | | | | | | | | | |!| | | }} | |||
{{familytree | |,|-|-|-|+|-|-|-|.| | | |,|-|-|^|-|-|-|.| }} | |||
{{familytree | C01 | | C02 | | C03 | | C04 | | | | | C05 | |C01=Reduced<br>mucosal blood flow|C02=Reduced<br> mucosal and<br> bicarbonate secreation|C03=Impaired<br>platelet aggregation|C04=Reduced<br>angiogenesis|C05=Increased<br>leucocyte adherence|}} | |||
{{familytree | |!| | | |!| | | |!| | | |!| | | | | | |!| | | }} | |||
{{familytree | |`|-|-|-|^|-|-|-|+|-|-|-|^|-|-|-|-|-|-|'| | | }} | |||
{{familytree | | | | | | | | | |!| | | | | | | | | | | | | | }} | |||
{{familytree | | | | | | | | | E01 | | | | | | | | | | | | |E01=Impaired defence<br>Impaired healing}} | |||
{{familytree | | | | | | | | | |!| | | | | | | | | | | | | | }} | |||
{{familytree | | | | | | | | | F01 | | | | | | | | | | | | |F01=Mucosal Injury}} | |||
{{familytree/end}} | |||
==Gross Pathology== | ===Gross and Microscopic Pathology=== | ||
* | {| class="wikitable" | ||
! colspan="2" | | |||
!Gross Pathology | |||
!Microscopic Pathology | |||
|- | |||
| colspan="2" |Varices | |||
|Large and tortuous veins that protrude into the lumen | |||
|Varices may be difficult to demonstrate in surgical specimens | |||
|- | |||
| colspan="2" |Mallory-Weiss Tear<ref name="pmid1465928">{{cite journal |vauthors=Renoult E, Biava MF, Aimone-Gastin I, Aouragh F, Hestin D, Kures L, Kessler M |title=Evolution and significance of Toxoplasma gondii antibody titers in kidney transplant recipients |journal=Transplant. Proc. |volume=24 |issue=6 |pages=2754–5 |year=1992 |pmid=1465928 |doi= |url=}}</ref> | |||
|Isolated or multiple cleft like mucosal defects | |||
| | |||
*Defects in the esophageal squamous mucosa. | |||
*Cells of acute inflammation. | |||
*Multiple ruptured blood vessels in the lamina propria or submucosa. | |||
*Prior lacerations may show various degrees of healing | |||
**Granulation tissue | |||
**Fibrosis<ref name="pmid1465928">{{cite journal |vauthors=Renoult E, Biava MF, Aimone-Gastin I, Aouragh F, Hestin D, Kures L, Kessler M |title=Evolution and significance of Toxoplasma gondii antibody titers in kidney transplant recipients |journal=Transplant. Proc. |volume=24 |issue=6 |pages=2754–5 |year=1992 |pmid=1465928 |doi= |url=}}</ref> | |||
**Epithelial regeneration. | |||
|- | |||
| rowspan="5" |Esophagitis<ref name="pmid24868280">{{cite journal |vauthors=Rosołowski M, Kierzkiewicz M |title=Etiology, diagnosis and treatment of infectious esophagitis |journal=Prz Gastroenterol |volume=8 |issue=6 |pages=333–7 |year=2013 |pmid=24868280 |pmc=4027832 |doi=10.5114/pg.2013.39914 |url=}}</ref> | |||
|Herpes esophagitis | |||
| | |||
* Shallow ulcers | |||
* Sharp and raised edges | |||
* Normal intervening erythematous mucosa | |||
|Ground glass inclusion bodies | |||
|- | |||
|Cytomegalovirus esophagitis | |||
| | |||
* Superficial ulcers | |||
* Well-circumscribed | |||
* CMV infects mesenchymal cells in the lamina propria and submucosa | |||
|Intranuclear inclusions | |||
|- | |||
|Fungal esophagitis | |||
| | |||
* Erythematous | |||
* Hyperemic | |||
* Friable | |||
* Discrete and raised white plaque | |||
|Neutrophils within the squamous epithelium | |||
|- | |||
|Pill esophagitis | |||
| | |||
* Discrete ulcers | |||
|Not specific and include | |||
* Necrosis | |||
* Prominent eosinophilic infiltrate | |||
* Spongiosis | |||
|- | |||
|Toxic esophagitis | |||
| | |||
* Mucosal erythema, | |||
* Edema | |||
* Hemorrhage | |||
* Necrosis | |||
|'''<u>Acid injury</u>''' | |||
* Coagulative necrosis | |||
* Eschar | |||
'''<u>Alkaline injury</u>''' | |||
* Liquefactive necrosis | |||
* Acute inflammation | |||
* Abundant granulation tissue | |||
|- | |||
| colspan="2" |Gastroesophageal | |||
Reflux Disease<ref name="pmid28943113">{{cite journal |vauthors=Pandit S, Boktor M, Alexander JS, Becker F, Morris J |title=Gastroesophageal reflux disease: A clinical overview for primary care physicians |journal=Pathophysiology |volume= |issue= |pages= |year=2017 |pmid=28943113 |doi=10.1016/j.pathophys.2017.09.001 |url=}}</ref> | |||
| | |||
| | |||
* Basal cell hyperplasia | |||
* Elongation of the lamina propria papillae | |||
* Mixed intraepithelial inflammation | |||
* Neutrophils, eosinophils, and lymphocytes | |||
* Squamous cell degeneration. | |||
|- | |||
| colspan="2" |Barrett Esophagus<ref name="pmid28501084">{{cite journal |vauthors=Rajendra S, Sharma P |title=Barrett Esophagus and Intramucosal Esophageal Adenocarcinoma |journal=Hematol. Oncol. Clin. North Am. |volume=31 |issue=3 |pages=409–426 |year=2017 |pmid=28501084 |doi=10.1016/j.hoc.2017.01.003 |url=}}</ref> | |||
| | |||
|Columnar metaplasia | |||
* Mucinous columnar cells | |||
* Goblet cells, and enterocyte-like cells, among others. | |||
* Cells of acute inflammation | |||
|- | |||
| colspan="2" |Acute Gastritis | |||
|Mucosal hyperemia associated with | |||
* Bleeding | |||
* Erosions | |||
* Ulcers | |||
| | |||
* Dilation and congestion of mucosal capillaries, edema, and hemorrhage in the lamina propria. | |||
* Ischemic-type changes such as | |||
** Degenerated and necrotic epithelium | |||
** Fibrinoid necrosis | |||
** Adherent fibrinopurulent debris | |||
|- | |||
| colspan="2" |Gastric Ulcers<ref name="pmid28798512">{{cite journal |vauthors=Drini M |title=Peptic ulcer disease and non-steroidal anti-inflammatory drugs |journal=Aust Prescr |volume=40 |issue=3 |pages=91–93 |year=2017 |pmid=28798512 |pmc=5478398 |doi=10.18773/austprescr.2017.037 |url=}}</ref> | |||
| | |||
* Solitary, typically less than 2 cm in diameter, and have sharply defined borders. | |||
* The ulcer edges are usually flat, and the base of the ulcer usually appears smooth. | |||
* The presence of a radiating pattern of rugal folds is characteristic of peptic ulcers | |||
| | |||
* Fibrinopurulent debris | |||
* Necrosis | |||
* Granulation tissue | |||
|- | |||
| colspan="2" |Portal Hypertensive Gastropathy<ref name="pmid26564121">{{cite journal |vauthors=Garg H, Gupta S, Anand AC, Broor SL |title=Portal hypertensive gastropathy and gastric antral vascular ectasia |journal=Indian J Gastroenterol |volume=34 |issue=5 |pages=351–8 |year=2015 |pmid=26564121 |doi=10.1007/s12664-015-0605-0 |url=}}</ref> | |||
| | |||
* Mosaic pattern of congestion | |||
* Most commonly involves the fundus | |||
| | |||
* Dilation, tortuosity, and thickening of small submucosal arteries and veins. | |||
* Mucosal capillaries may also show congestion, dilation, and proliferation. | |||
|- | |||
| colspan="2" |Gastric Antral Vascular Ectasia<ref name="pmid26564121">{{cite journal |vauthors=Garg H, Gupta S, Anand AC, Broor SL |title=Portal hypertensive gastropathy and gastric antral vascular ectasia |journal=Indian J Gastroenterol |volume=34 |issue=5 |pages=351–8 |year=2015 |pmid=26564121 |doi=10.1007/s12664-015-0605-0 |url=}}</ref> | |||
|Linear pattern of mucosal congestion in the antrum termed “watermelon stomach | |||
|'''<u>Antral biopsies</u>''' show | |||
* Congestion | |||
* Dilated mucosal capillaries | |||
* Vascular microthrombi | |||
The mucosa also shows | |||
* Foveolar hyperplasia | |||
* Fibromuscular hyperplasia | |||
* Edema and regenerative changes | |||
|- | |||
| colspan="2" |Reactive (Chemical) Gastropathy | |||
|'''<u>Stomach</u>''' | |||
* Edema | |||
* Surface erosions | |||
* Polypoid changes, and friability | |||
|The mucosa shows | |||
* Congestion | |||
* Edema | |||
* Fibromuscular hyperplasia | |||
* Foveolar hyperplasia | |||
|- | |||
| colspan="2" |Peptic Disease | |||
|Wide range of findings | |||
* From normal/slightly edematous mucosa to increased friability, erosions, and ulcers | |||
| | |||
* Increased plasma cells | |||
* Neutrophilic infiltrate | |||
* Reactive epithelial changes, including villous blunting. | |||
* The surface epithelium usually shows mucous cell (pseudopyloric) metaplasia | |||
|- | |||
| colspan="2" |Ischemia | |||
|Hypoperfused ulcers | |||
|'''<u>Acute ischemia</u>''' | |||
* Mucosal edema | |||
* Congestion | |||
* Superficial necrosis | |||
* Coagulative necrosis | |||
'''<u>Chronic ischemia</u>''' | |||
* Fibrosis | |||
* Strictures | |||
|- | |||
| colspan="2" |Structural Abnormalities of Blood Vessels<ref name="pmid11355900">{{cite journal |vauthors=Gordon FH, Watkinson A, Hodgson H |title=Vascular malformations of the gastrointestinal tract |journal=Best Pract Res Clin Gastroenterol |volume=15 |issue=1 |pages=41–58 |year=2001 |pmid=11355900 |doi=10.1053/bega.2000.0155 |url=}}</ref> | |||
|Large-caliber artery within the submucosa | |||
|Dilated venules and arteriole in direct communication with each other | |||
|- | |||
| colspan="2" |Inflammatory Bowel Disease | |||
| | |||
|Lymphoplasmacytic infiltrate with numerous neutrophils | |||
|} | |||
==References== | ==References== | ||
Revision as of 18:28, 3 November 2017
|
Upper gastrointestinal bleeding Microchapters |
|
Differentiating Upper Gastrointestinal Bleeding from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Management |
|
Surgery |
|
Case Studies |
|
Upper gastrointestinal bleeding pathophysiology On the Web |
|
American Roentgen Ray Society Images of Upper gastrointestinal bleeding pathophysiology |
|
Directions to Hospitals Treating Upper gastrointestinal bleeding |
|
Risk calculators and risk factors for Upper gastrointestinal bleeding pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief:
Overview
The exact pathogenesis of [disease name] is not fully understood.
OR
It is thought that [disease name] is the result of / is mediated by / is produced by / is caused by either [hypothesis 1], [hypothesis 2], or [hypothesis 3].
OR
[Pathogen name] is usually transmitted via the [transmission route] route to the human host.
OR
Following transmission/ingestion, the [pathogen] uses the [entry site] to invade the [cell name] cell.
OR
[Disease or malignancy name] arises from [cell name]s, which are [cell type] cells that are normally involved in [function of cells].
OR
The progression to [disease name] usually involves the [molecular pathway].
OR
The pathophysiology of [disease/malignancy] depends on the histological subtype.
Pathophysiology
Blood supply of Foregut
The digestive system is supplied by the celiac artery. The celiac artery is the first major branch from the abdominal aorta, and is the only major artery that nourishes the digestive organs.[1][2][3][4][5][6][7]
| Foregut | Blood supply | |
|---|---|---|
| Esophagus |
Upper esophageal sphincter |
Inferior thyroid artery |
| Thoracic esophagus | Aortic esophageal arteries or branches of the bronchial arteries | |
|
Distal esophagus |
Left gastric artery and left phrenic artery | |
| Stomach | Lesser curvature | Right and left gastric arteries |
| Greater curvature | Right and left gastroepiploic arteries | |
| Gastric fundus | Short gastric arteries | |
| Duodenum | First and second parts |
Gastroduodenal artery (GDA) and |
| Third and fourth parts | Inferior pancreaticoduodenal artery | |

Source: By Mikael Häggström.https://commons.wikimedia.org/w/index.php?curid=3416062
Mucosal barrier
- The gastric mucosa is protected from the acidic environment by mucus, bicarbonate, prostaglandins, and blood flow.[8][9][10]
- This mucosal barrier consists of three protective components which include:
- Layer of epithelial cell lining.
- Layer of mucus, secreted by surface epithelial cells and foveolar cells.
- Layer of bicarbonate ions, secreted by the surface epithelial cells.
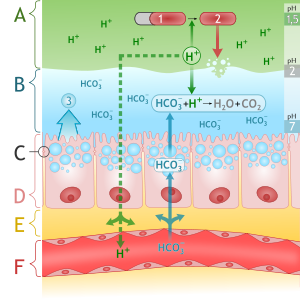
Source: By M•Komorniczak (http://creativecommons.org/licenses/by/3.0)], via Wikimedia Commons
The following table demonstrates the defense mechanisms of gastric mucosal barrier[11]
| Defense mechanisms of gastric mucosal barrier | |
|---|---|
| Mucus layer | Forms a protective gel-like coating over the entire gastric mucosal surface |
| Epithelial layer | Epithelial cell layer are bound by tight junctions that repel fluids |
| Bicarbonate ions | Neutralize acids |
Pathogenesis
The main inciting event in the pathogeneis of upper GI bleeding is damage to mucosal injury. This mucosal injury can occur at various levels of GI tract. If the damage and bleeding is confined up to ligament of Treitz, it is defined as upper GI bleeding.[12][13]
| Etiology | Frequency of occurance |
|---|---|
| Peptic ulcer disease | 50% |
| Variceal bleeding | 20% |
| Esophagitis, gastritis, and duodenitis | 10-15% |
| Mallory-Weiss tear | 15% |
| Malignancy | 3-5% |
| Arteriovenous malformation | <3% |
| Gastric antral vascular ectasia | <1% |
| Dieulafoy lesion | <1% |
Pathogenesis
- Regardless of etiology, if the balance of gastric acid secretion and mucosal defenses is disrupted, acid interacts with the epithelium to cause damage.[14][15][16]
- Varices are large, tortuous veins and protrude into the lumen, rupturing.[17]
- Helicobacter pylori disrupts the mucosal barrier and causes inflammation of the mucosa of the stomach and duodenum.[18][19]
- As the ulcer progresses beyond the mucosa to the submucosa the inflammation causes weakening and necrosis of arterial walls, leading to pseudoaneurysm formation followed by rupture and hemorrhage.[20]
- NSAIDs inhibit cyclooxygenase, leading to impaired mucosal defenses by decreasing mucosal prostaglandin synthesis.[21]
- During stress, there is acid hypersecretion; therefore, the breakdown of mucosal defenses leads to injury of the mucosa and subsequent bleeding.
- Mucosal defects along with dilated and tortuous vessels in dieulafoy lesion put them at risk for rupture because of necrosis of the arterial wall from exposure to gastric acid.[22][23][24][25]
| NSAIDS | |||||||||||||||||||||||||||||||||||||||||||||||
| Inhibits cycloxygenase pathway | |||||||||||||||||||||||||||||||||||||||||||||||
| COX-1 | COX-2 | ||||||||||||||||||||||||||||||||||||||||||||||
| Reduced mucosal blood flow | Reduced mucosal and bicarbonate secreation | Impaired platelet aggregation | Reduced angiogenesis | Increased leucocyte adherence | |||||||||||||||||||||||||||||||||||||||||||
| Impaired defence Impaired healing | |||||||||||||||||||||||||||||||||||||||||||||||
| Mucosal Injury | |||||||||||||||||||||||||||||||||||||||||||||||
Gross and Microscopic Pathology
| Gross Pathology | Microscopic Pathology | ||
|---|---|---|---|
| Varices | Large and tortuous veins that protrude into the lumen | Varices may be difficult to demonstrate in surgical specimens | |
| Mallory-Weiss Tear[26] | Isolated or multiple cleft like mucosal defects |
| |
| Esophagitis[27] | Herpes esophagitis |
|
Ground glass inclusion bodies |
| Cytomegalovirus esophagitis |
|
Intranuclear inclusions | |
| Fungal esophagitis |
|
Neutrophils within the squamous epithelium | |
| Pill esophagitis |
|
Not specific and include
| |
| Toxic esophagitis |
|
Acid injury
Alkaline injury
| |
| Gastroesophageal
Reflux Disease[28] |
| ||
| Barrett Esophagus[29] | Columnar metaplasia
| ||
| Acute Gastritis | Mucosal hyperemia associated with
|
| |
| Gastric Ulcers[30] |
|
| |
| Portal Hypertensive Gastropathy[31] |
|
| |
| Gastric Antral Vascular Ectasia[31] | Linear pattern of mucosal congestion in the antrum termed “watermelon stomach | Antral biopsies show
The mucosa also shows
| |
| Reactive (Chemical) Gastropathy | Stomach
|
The mucosa shows
| |
| Peptic Disease | Wide range of findings
|
| |
| Ischemia | Hypoperfused ulcers | Acute ischemia
Chronic ischemia
| |
| Structural Abnormalities of Blood Vessels[32] | Large-caliber artery within the submucosa | Dilated venules and arteriole in direct communication with each other | |
| Inflammatory Bowel Disease | Lymphoplasmacytic infiltrate with numerous neutrophils | ||
References
- ↑ Feldman SE (1970). "Blood supply to stomach". Calif Med. 112 (4): 55. PMC 1501289. PMID 18730308.
- ↑ Granger DN, Holm L, Kvietys P (2015). "The Gastrointestinal Circulation: Physiology and Pathophysiology". Compr Physiol. 5 (3): 1541–83. doi:10.1002/cphy.c150007. PMID 26140727.
- ↑ Geboes K, Geboes KP, Maleux G (2001). "Vascular anatomy of the gastrointestinal tract". Best Pract Res Clin Gastroenterol. 15 (1): 1–14. doi:10.1053/bega.2000.0152. PMID 11355897.
- ↑ Varga F, Csáky TZ (1976). "Changes in the blood supply of the gastrointestinal tract in rats with age". Pflugers Arch. 364 (2): 129–33. PMID 986621.
- ↑ Matuchansky C, Bernier JJ (1973). "[Prostaglandins and the digestive tract]". Biol Gastroenterol (Paris) (in French). 6 (3): 251–68. PMID 4599528.
- ↑ Radbil' OS (1974). "[Prostaglandins and the digestive system organs]". Ter. Arkh. (in Russian). 46 (4): 6–14. PMID 4372738.
- ↑ Robert A (1980). "Prostaglandins and digestive diseases". Adv Prostaglandin Thromboxane Res. 8: 1533–41. PMID 6990725.
- ↑ Hills BA, Butler BD, Lichtenberger LM (1983). "Gastric mucosal barrier: hydrophobic lining to the lumen of the stomach". Am. J. Physiol. 244 (5): G561–8. PMID 6846549.
- ↑ Clamp JR, Ene D (1989). "The gastric mucosal barrier". Methods Find Exp Clin Pharmacol. 11 Suppl 1: 19–25. PMID 2657286.
- ↑ Werther JL (2000). "The gastric mucosal barrier". Mt. Sinai J. Med. 67 (1): 41–53. PMID 10677782.
- ↑ Forssell H (1988). "Gastric mucosal defence mechanisms: a brief review". Scand. J. Gastroenterol. Suppl. 155: 23–8. PMID 3072665.
- ↑ van Leerdam ME (2008). "Epidemiology of acute upper gastrointestinal bleeding". Best Pract Res Clin Gastroenterol. 22 (2): 209–24. doi:10.1016/j.bpg.2007.10.011. PMID 18346679.
- ↑ Boonpongmanee S, Fleischer DE, Pezzullo JC, Collier K, Mayoral W, Al-Kawas F, Chutkan R, Lewis JH, Tio TL, Benjamin SB (2004). "The frequency of peptic ulcer as a cause of upper-GI bleeding is exaggerated". Gastrointest. Endosc. 59 (7): 788–94. PMID 15173790.
- ↑ Gartner AH (1976). "Aspirin-induced gastritis and gastrointestinal bleeding". J Am Dent Assoc. 93 (1): 111–7. PMID 6499.
- ↑ Iwamoto J, Saito Y, Honda A, Matsuzaki Y (2013). "Clinical features of gastroduodenal injury associated with long-term low-dose aspirin therapy". World J. Gastroenterol. 19 (11): 1673–82. doi:10.3748/wjg.v19.i11.1673. PMC 3607744. PMID 23555156.
- ↑ Hawkey CJ (1996). "Non-steroidal anti-inflammatory drug gastropathy: causes and treatment". Scand. J. Gastroenterol. Suppl. 220: 124–7. PMID 8898449.
- ↑ Quan S, Yang H, Tanyingoh D, Villeneuve PJ, Stieb DM, Johnson M, Hilsden R, Madsen K, van Zanten SV, Novak K, Lang E, Ghosh S, Kaplan GG (2015). "Upper gastrointestinal bleeding due to peptic ulcer disease is not associated with air pollution: a case-crossover study". BMC Gastroenterol. 15: 131. doi:10.1186/s12876-015-0363-6. PMC 4604641. PMID 26467538.
- ↑ Quan, C (2002). "Management of peptic ulcer disease not related to Helicobacter pylori or NSAIDs". The American Journal of Gastroenterology. 97 (12): 2950–2961. doi:10.1016/S0002-9270(02)05485-0. ISSN 0002-9270.
- ↑ Malfertheiner, Peter; Chan, Francis KL; McColl, Kenneth EL (2009). "Peptic ulcer disease". The Lancet. 374 (9699): 1449–1461. doi:10.1016/S0140-6736(09)60938-7. ISSN 0140-6736.
- ↑ Quan S, Frolkis A, Milne K, Molodecky N, Yang H, Dixon E, Ball CG, Myers RP, Ghosh S, Hilsden R, van Zanten SV, Kaplan GG (2014). "Upper-gastrointestinal bleeding secondary to peptic ulcer disease: incidence and outcomes". World J. Gastroenterol. 20 (46): 17568–77. doi:10.3748/wjg.v20.i46.17568. PMC 4265619. PMID 25516672.
- ↑ Xi B, Jia JJ, Lin BY, Geng L, Zheng SS (2016). "Peptic ulcers accompanied with gastrointestinal bleeding, pylorus obstruction and cholangitis secondary to choledochoduodenal fistula: A case report". Oncol Lett. 11 (1): 481–483. doi:10.3892/ol.2015.3908. PMC 4727103. PMID 26870237.
- ↑ Stern AI, Korman MG, Hunt PS, Hansky J, Hillman HS, Schmidt GT (1979). "The Mallory-Weiss lesion as a cause of upper gastrointestinal bleeding". Aust N Z J Surg. 49 (1): 13–8. PMID 313784.
- ↑ Katz PO, Salas L (1993). "Less frequent causes of upper gastrointestinal bleeding". Gastroenterol. Clin. North Am. 22 (4): 875–89. PMID 8307643.
- ↑ Sabljak P, Velicković D, Stojakov D, Bjelović M, Ebrahimi K, Spica B, Sljukić V, Pesko P (2007). "[Less frequent causes of upper gastrointestinal bleeding]". Acta Chir Iugosl. 54 (1): 119–23. PMID 17633871.
- ↑ Depolo A, Dobrila-Dintinjana R, Uravi M, Grbas H, Rubini M (2001). "[Upper gastrointestinal bleeding - Review of our ten years results]". Zentralbl Chir (in German). 126 (10): 772–6. doi:10.1055/s-2001-18265. PMID 11727185.
- ↑ 26.0 26.1 Renoult E, Biava MF, Aimone-Gastin I, Aouragh F, Hestin D, Kures L, Kessler M (1992). "Evolution and significance of Toxoplasma gondii antibody titers in kidney transplant recipients". Transplant. Proc. 24 (6): 2754–5. PMID 1465928.
- ↑ Rosołowski M, Kierzkiewicz M (2013). "Etiology, diagnosis and treatment of infectious esophagitis". Prz Gastroenterol. 8 (6): 333–7. doi:10.5114/pg.2013.39914. PMC 4027832. PMID 24868280.
- ↑ Pandit S, Boktor M, Alexander JS, Becker F, Morris J (2017). "Gastroesophageal reflux disease: A clinical overview for primary care physicians". Pathophysiology. doi:10.1016/j.pathophys.2017.09.001. PMID 28943113.
- ↑ Rajendra S, Sharma P (2017). "Barrett Esophagus and Intramucosal Esophageal Adenocarcinoma". Hematol. Oncol. Clin. North Am. 31 (3): 409–426. doi:10.1016/j.hoc.2017.01.003. PMID 28501084.
- ↑ Drini M (2017). "Peptic ulcer disease and non-steroidal anti-inflammatory drugs". Aust Prescr. 40 (3): 91–93. doi:10.18773/austprescr.2017.037. PMC 5478398. PMID 28798512.
- ↑ 31.0 31.1 Garg H, Gupta S, Anand AC, Broor SL (2015). "Portal hypertensive gastropathy and gastric antral vascular ectasia". Indian J Gastroenterol. 34 (5): 351–8. doi:10.1007/s12664-015-0605-0. PMID 26564121.
- ↑ Gordon FH, Watkinson A, Hodgson H (2001). "Vascular malformations of the gastrointestinal tract". Best Pract Res Clin Gastroenterol. 15 (1): 41–58. doi:10.1053/bega.2000.0155. PMID 11355900.