Small cell carcinoma of the lung pathophysiology: Difference between revisions
No edit summary |
|||
| Line 4: | Line 4: | ||
{{CMG}}; {{AE}} {{AL}} | {{CMG}}; {{AE}} {{AL}} | ||
==Overview== | ==Overview== | ||
Small cell lung cancer | Small cell lung cancer is the most aggressive form of [[lung cancer]] and has the highest association with [[smoking]] of all lung cancers. Small cell lung cancer usually starts in the [[bronchi]] and expands through the [[bronchial]] mucosa. Small cell lung cancer often [[metastasize]]s rapidly to other parts of the body, including the [[brain]], [[liver]], and [[bone]]. A mutation in the [[p53]] gene is reported in 75%-100% of the cases. Other molecular abnormalities that contribute to the development of small cell lung cancer have been described. | ||
==Pathogenesis== | ==Pathogenesis== | ||
=== | ===Genetics=== | ||
* A mutation in the [[p53]] gene is reported in 75%-100% of the cases of | * A mutation in the [[p53]] gene is reported in 75%-100% of the cases of small cell lung cancer. Other molecular abnormalities that contribute to the development of small cell lung cancer have been described, such as:<ref>{{Cite journal | ||
| author = [[Grace K. Dy]] & [[Alex A. Adjei]] | | author = [[Grace K. Dy]] & [[Alex A. Adjei]] | ||
| title = Novel targets for lung cancer therapy: part I | | title = Novel targets for lung cancer therapy: part I | ||
| Line 26: | Line 26: | ||
** [[RB1]] deletion (loss of [[RB1]] protein) | ** [[RB1]] deletion (loss of [[RB1]] protein) | ||
** [[VEGF]] ([[vascular endothelial growth factor]]) expression | ** [[VEGF]] ([[vascular endothelial growth factor]]) expression | ||
** [[c-kit]]/[[Stem cell factor|SCFR]] ([[stem cell factor]] receptor) coexpression (in 70% of cases): tumor cells of | ** [[c-kit]]/[[Stem cell factor|SCFR]] ([[stem cell factor]] receptor) coexpression (in 70% of cases): tumor cells of small cell lung cancer express and secrete the [[stem cell factor]] which binds to [[c-kit]].<ref>{{Cite journal | ||
| author = [[K. Hibi]], [[T. Takahashi]], [[Y. Sekido]], [[R. Ueda]], [[T. Hida]], [[Y. Ariyoshi]], [[H. Takagi]] & [[T. Takahashi]] | | author = [[K. Hibi]], [[T. Takahashi]], [[Y. Sekido]], [[R. Ueda]], [[T. Hida]], [[Y. Ariyoshi]], [[H. Takagi]] & [[T. Takahashi]] | ||
| title = Coexpression of the stem cell factor and the c-kit genes in small-cell lung cancer | | title = Coexpression of the stem cell factor and the c-kit genes in small-cell lung cancer | ||
| Line 38: | Line 38: | ||
}}</ref> | }}</ref> | ||
* There are 4 [[tumor suppressor genes]] that are affected with [[chromosome 3]]p deletion. These [[tumor suppressor genes]] play a role in the development of | * There are 4 [[tumor suppressor genes]] that are affected with [[chromosome 3]]p deletion. These [[tumor suppressor genes]] play a role in the development of small cell lung cancer. The genes are: | ||
** [[Fragile histidine triad gene]] ([[FHIT]]): the FHIT gene encodes an enzyme called diadenosine triphosphate hydrolase and plays a role in the control of the [[cell cycle]] control and proapoptosis.<ref>{{Cite journal | ** [[Fragile histidine triad gene]] ([[FHIT]]): the FHIT gene encodes an enzyme called diadenosine triphosphate hydrolase and plays a role in the control of the [[cell cycle]] control and proapoptosis.<ref>{{Cite journal | ||
| author = [[Yuri Pekarsky]], [[Alexey Palamarchuk]], [[Kay Huebner]] & [[Carlo M. Croce]] | | author = [[Yuri Pekarsky]], [[Alexey Palamarchuk]], [[Kay Huebner]] & [[Carlo M. Croce]] | ||
| Line 148: | Line 148: | ||
===Paraneoplastic Syndrome=== | ===Paraneoplastic Syndrome=== | ||
* | * Small cell lung cancer is one of the most common tumors associated with a [[paraneoplastic syndrome]]. The most common [[endocrine]] condition associated with small cell lung cancer is syndrome of inappropriate antidiuretic hormone ([[SIADH]]), where there is an excessive secretion of antidueretic hormone ([[ADH]]) which leads to [[hyponatremia]]. | ||
* Other conditions that are related to | * Other conditions that are related to small cell lung cancer are: | ||
** Production of [[atrial natriuretic peptide]] ([[ANP]]) leading to [[hyponatremia]], [[natriuresis]] and [[hypotension]] | ** Production of [[atrial natriuretic peptide]] ([[ANP]]) leading to [[hyponatremia]], [[natriuresis]] and [[hypotension]] | ||
** Ectopic [[ACTH]] production, which causes [[Cushing syndrome]] | ** Ectopic [[ACTH]] production, which causes [[Cushing syndrome]] | ||
| Line 167: | Line 167: | ||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
In | In small cell lung cancer, the tumor cells are small and round, but they can sometimes be ovoid or spindle shaped. They have a scant [[cytoplasm]] with a high mitotic count and a hyperchromatic [[nuclei]]. Nearly all small cell lung cancer are immunoreactive for [[keratin]], [[thyroid transcription factor 1]], and epithelial membrane antigen. Neuroendocrine and neural differentiation result in the expression of molecules like dopa decarboxylase, [[calcitonin]], neuron-specific [[enolase]], [[chromogranin A]], [[CD56]] (also known as nucleosomal histone kinase 1 or neural-cell adhesion molecule), gastrin-releasing peptide, and [[insulin-like growth factor 1]]. One or more markers of neuroendocrine differentiation can be found in approximately 75% of small cell lung cancer.<ref name="NCI"> National Cancer Institute: PDQ® Small Cell Lung Cancer Treatment. Bethesda, MD: National Cancer Institute. Available at: http://www.cancer.gov/cancertopics/pdq/treatment/small-cell-lung/healthprofessional.</ref> | ||
<div align="left"> | <div align="left"> | ||
Revision as of 15:34, 23 November 2015
|
Small Cell Carcinoma of the Lung Microchapters |
|
Differentiating Small Cell Carcinoma of the Lung from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Small cell carcinoma of the lung pathophysiology On the Web |
|
American Roentgen Ray Society Images of Small cell carcinoma of the lung pathophysiology |
|
Small cell carcinoma of the lung pathophysiology in the news |
|
Directions to Hospitals Treating Small cell carcinoma of the lung |
|
Risk calculators and risk factors for Small cell carcinoma of the lung pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alejandro Lemor, M.D. [2]
Overview
Small cell lung cancer is the most aggressive form of lung cancer and has the highest association with smoking of all lung cancers. Small cell lung cancer usually starts in the bronchi and expands through the bronchial mucosa. Small cell lung cancer often metastasizes rapidly to other parts of the body, including the brain, liver, and bone. A mutation in the p53 gene is reported in 75%-100% of the cases. Other molecular abnormalities that contribute to the development of small cell lung cancer have been described.
Pathogenesis
Genetics
- A mutation in the p53 gene is reported in 75%-100% of the cases of small cell lung cancer. Other molecular abnormalities that contribute to the development of small cell lung cancer have been described, such as:[1]
- Chromosome 3p deletion (in 90% of cases)
- MYC amplification (in 30% of cases)
- BCL2 expression (in 95% of cases)
- GRP (gastrin-releasing peptide) expression
- RB1 deletion (loss of RB1 protein)
- VEGF (vascular endothelial growth factor) expression
- c-kit/SCFR (stem cell factor receptor) coexpression (in 70% of cases): tumor cells of small cell lung cancer express and secrete the stem cell factor which binds to c-kit.[2]
- There are 4 tumor suppressor genes that are affected with chromosome 3p deletion. These tumor suppressor genes play a role in the development of small cell lung cancer. The genes are:
- Fragile histidine triad gene (FHIT): the FHIT gene encodes an enzyme called diadenosine triphosphate hydrolase and plays a role in the control of the cell cycle control and proapoptosis.[3]
- RASS effector homologue (RASSF1): RASSF1 stabilizes the cell cycle and induces G2-M arrest, preventing cells from rapidly growing.[4]
- Retinoic acid receptor beta
- FUS1
Smoking
- The association between smoking and lung cancer is well established. In fact, studies from the early 1950's demonstrated an elevated risk of lung cancer with smoking.[5][6] In particular, small cell lung cancer is highly associated with smoking, even more than the other types of lung cancer.[7]
- There are more than 60 carcinogens in a cigarette,[8] including radioisotopes from the radon decay sequence, nitrosamine, and benzopyrene. These carcinogens cause oxidative stress through reactive oxygen species and promote point mutations in different genes, especially in TP53 and KRAS. These events culminate in the development of neoplastic cells.[9][10] In addition, nicotine appears to depress the immune response to malignant growth in exposed tissues.
Radon Exposure
- There are several studies on the association between the exposure to radon and lung cancer.[11][12][13] Radon damages the epithelial lining of the lung by either interacting directly with the cellular DNA and causing chromosomal damage and gene mutations, or indirectly through the effect of free radicals. Radon gas emits alpha particles that react with the water molecules, creating several reactive oxygen species that react with other molecules and cause biologic damage to the lung cells.[14][15][16]
Paraneoplastic Syndrome
- Small cell lung cancer is one of the most common tumors associated with a paraneoplastic syndrome. The most common endocrine condition associated with small cell lung cancer is syndrome of inappropriate antidiuretic hormone (SIADH), where there is an excessive secretion of antidueretic hormone (ADH) which leads to hyponatremia.
- Other conditions that are related to small cell lung cancer are:
- Production of atrial natriuretic peptide (ANP) leading to hyponatremia, natriuresis and hypotension
- Ectopic ACTH production, which causes Cushing syndrome
- Lambert-Eaton syndrome due to the production of antibodies directed against the antigens of the neuromuscular junctions
Gross Pathology
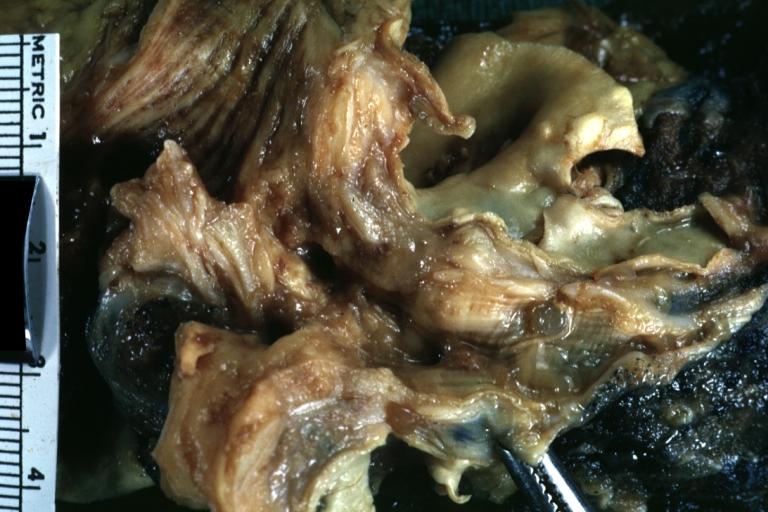
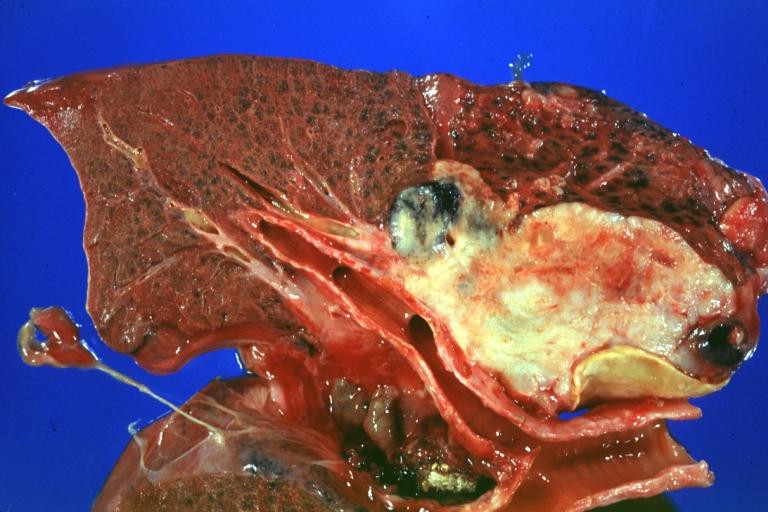
-
Gross fixed tissue opened bronchus at hilum showing tumor close-up.
-
Gross natural color photo of left upper lobe neoplasm extending into mediastinal pleura and surrounding portion of aorta node metastasis easily seen small cell carcinoma (unusual spindle cell areas)
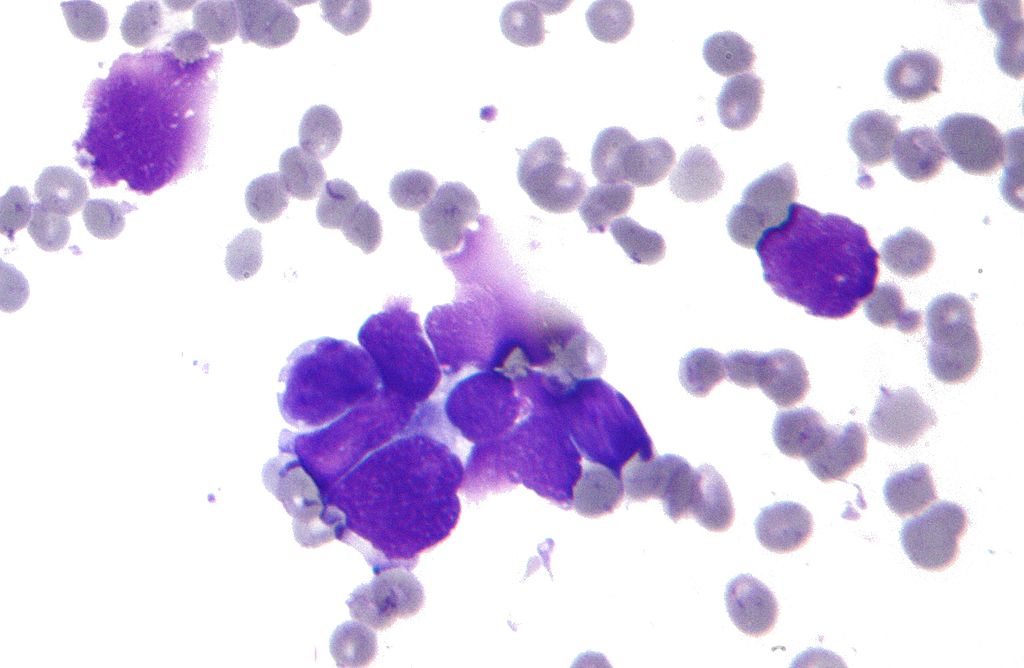
Microscopic Pathology
In small cell lung cancer, the tumor cells are small and round, but they can sometimes be ovoid or spindle shaped. They have a scant cytoplasm with a high mitotic count and a hyperchromatic nuclei. Nearly all small cell lung cancer are immunoreactive for keratin, thyroid transcription factor 1, and epithelial membrane antigen. Neuroendocrine and neural differentiation result in the expression of molecules like dopa decarboxylase, calcitonin, neuron-specific enolase, chromogranin A, CD56 (also known as nucleosomal histone kinase 1 or neural-cell adhesion molecule), gastrin-releasing peptide, and insulin-like growth factor 1. One or more markers of neuroendocrine differentiation can be found in approximately 75% of small cell lung cancer.[17]
-
Histopathologic image of small cell carcinoma of the lung. CT-guided core needle biopsy. H & E stain.
-
Micrograph of a small-cell carcinoma of the lung showing cells with nuclear moulding, minimal amount of cytoplasm and stippled chromatin. FNA specimen. Field stain.
-
Anaplastic (microcellular, oat cell) carcinoma from the lung.
-
Histopathologic image of small-cell carcinoma of the lung. CT-guided core needle biopsy.
-
Small cell carcinoma, pleural FNA.
References
- ↑ Grace K. Dy & Alex A. Adjei (2002). "Novel targets for lung cancer therapy: part I". Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 20 (12): 2881–2894. PMID 12065566. Unknown parameter
|month=ignored (help) - ↑ K. Hibi, T. Takahashi, Y. Sekido, R. Ueda, T. Hida, Y. Ariyoshi, H. Takagi & T. Takahashi (1991). "Coexpression of the stem cell factor and the c-kit genes in small-cell lung cancer". Oncogene. 6 (12): 2291–2296. PMID 1722571. Unknown parameter
|month=ignored (help) - ↑ Yuri Pekarsky, Alexey Palamarchuk, Kay Huebner & Carlo M. Croce (2002). "FHIT as tumor suppressor: mechanisms and therapeutic opportunities". Cancer biology & therapy. 1 (3): 232–236. PMID 12432269. Unknown parameter
|month=ignored (help) - ↑ Rong Rong, Weixin Jin, Jennifer Zhang, M. Saeed Sheikh & Ying Huang (2004). "Tumor suppressor RASSF1A is a microtubule-binding protein that stabilizes microtubules and induces G2/M arrest". Oncogene. 23 (50): 8216–8230. doi:10.1038/sj.onc.1207901. PMID 15378022. Unknown parameter
|month=ignored (help) - ↑ R. DOLL & A. B. HILL (1950). "Smoking and carcinoma of the lung; preliminary report". British medical journal. 2 (4682): 739–748. PMID 14772469. Unknown parameter
|month=ignored (help) - ↑ Peter N. Lee, Barbara A. Forey & Katharine J. Coombs (2012). "Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer". BMC cancer. 12: 385. doi:10.1186/1471-2407-12-385. PMID 22943444.
- ↑ NCCN Clinical Practice Guidelines in Oncology. Small Cell Lung Cancer, version 2.2014
- ↑ Hecht, S (Oct 2003). "Tobacco carcinogens, their biomarkers and tobacco-induced cancer". Nature Reviews. Cancer. Nature Publishing Group. 3 (10): 733–744. doi:10.1038/nrc1190. PMID 14570033. Retrieved 2007-08-10.
- ↑ Pleasance, Erin D.; Stephens, Philip J.; O’Meara, Sarah; McBride, David J.; Meynert, Alison; Jones, David; Lin, Meng-Lay; Beare, David; Lau, King Wai; Greenman, Chris; Varela, Ignacio; Nik-Zainal, Serena; Davies, Helen R.; Ordoñez, Gonzalo R.; Mudie, Laura J.; Latimer, Calli; Edkins, Sarah; Stebbings, Lucy; Chen, Lina; Jia, Mingming; Leroy, Catherine; Marshall, John; Menzies, Andrew; Butler, Adam; Teague, Jon W.; Mangion, Jonathon; Sun, Yongming A.; McLaughlin, Stephen F.; Peckham, Heather E.; Tsung, Eric F.; Costa, Gina L.; Lee, Clarence C.; Minna, John D.; Gazdar, Adi; Birney, Ewan; Rhodes, Michael D.; McKernan, Kevin J.; Stratton, Michael R.; Futreal, P. Andrew; Campbell, Peter J. (2009). "A small-cell lung cancer genome with complex signatures of tobacco exposure". Nature. 463 (7278): 184–190. doi:10.1038/nature08629. ISSN 0028-0836.
- ↑ David M. DeMarini (2004). "Genotoxicity of tobacco smoke and tobacco smoke condensate: a review". Mutation research. 567 (2–3): 447–474. doi:10.1016/j.mrrev.2004.02.001. PMID 15572290. Unknown parameter
|month=ignored (help) - ↑ S. Darby, D. Hill, A. Auvinen, J. M. Barros-Dios, H. Baysson, F. Bochicchio, H. Deo, R. Falk, F. Forastiere, M. Hakama, I. Heid, L. Kreienbrock, M. Kreuzer, F. Lagarde, I. Makelainen, C. Muirhead, W. Oberaigner, G. Pershagen, A. Ruano-Ravina, E. Ruosteenoja, A. Schaffrath Rosario, M. Tirmarche, L. Tomasek, E. Whitley, H.-E. Wichmann & R. Doll (2005). "Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies". BMJ (Clinical research ed.). 330 (7485): 223. doi:10.1136/bmj.38308.477650.63. PMID 15613366. Unknown parameter
|month=ignored (help) - ↑ J. H. Lubin, J. D. Jr Boice, C. Edling, R. W. Hornung, G. R. Howe, E. Kunz, R. A. Kusiak, H. I. Morrison, E. P. Radford & J. M. Samet (1995). "Lung cancer in radon-exposed miners and estimation of risk from indoor exposure". Journal of the National Cancer Institute. 87 (11): 817–827. PMID 7791231. Unknown parameter
|month=ignored (help) - ↑ B. Grosche, M. Kreuzer, M. Kreisheimer, M. Schnelzer & A. Tschense (2006). "Lung cancer risk among German male uranium miners: a cohort study, 1946-1998". British journal of cancer. 95 (9): 1280–1287. doi:10.1038/sj.bjc.6603403. PMID 17043686. Unknown parameter
|month=ignored (help) - ↑ Harley, N. H.; Chittaporn, P.; Heikkinen, M. S. A.; Meyers, O. A.; Robbins, E. S. (2008). "Radon carcinogenesis: risk data and cellular hits". Radiation Protection Dosimetry. 130 (1): 107–109. doi:10.1093/rpd/ncn123. ISSN 0144-8420.
- ↑ Al-Zoughool, Mustafa; Krewski, Daniel (2009). "Health effects of radon: A review of the literature". International Journal of Radiation Biology. 85 (1): 57–69. doi:10.1080/09553000802635054. ISSN 0955-3002.
- ↑ Michael C. R. Alavanja (2002). "Biologic damage resulting from exposure to tobacco smoke and from radon: implication for preventive interventions". Oncogene. 21 (48): 7365–7375. doi:10.1038/sj.onc.1205798. PMID 12379879. Unknown parameter
|month=ignored (help) - ↑ National Cancer Institute: PDQ® Small Cell Lung Cancer Treatment. Bethesda, MD: National Cancer Institute. Available at: http://www.cancer.gov/cancertopics/pdq/treatment/small-cell-lung/healthprofessional.