Methylnaltrexone: Difference between revisions
m (Protected "Methylnaltrexone": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
Rabin Bista (talk | contribs) No edit summary |
||
| Line 26: | Line 26: | ||
| routes_of_administration = | | routes_of_administration = | ||
}} | }} | ||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | |||
== Overview == | |||
'''Methylnaltrexone''' ('''MTNX''') is one of the newer agents of peripherally-acting [[Mu opioid receptor|μ-opioid]] [[Receptor antagonist|antagonists]] that act to reverse some of the side effects of [[opioid]] drugs such as [[constipation]] without affecting [[analgesia]] or precipitating [[withdrawal]]s. Because it contains a permanently charged tetravalent nitrogen atom, it cannot cross the [[blood-brain barrier]], and so has antagonist effects throughout the body, counteracting effects such as [[itching]] and constipation, but without affecting opioid effects in the brain such as analgesia. | '''Methylnaltrexone''' ('''MTNX''') is one of the newer agents of peripherally-acting [[Mu opioid receptor|μ-opioid]] [[Receptor antagonist|antagonists]] that act to reverse some of the side effects of [[opioid]] drugs such as [[constipation]] without affecting [[analgesia]] or precipitating [[withdrawal]]s. Because it contains a permanently charged tetravalent nitrogen atom, it cannot cross the [[blood-brain barrier]], and so has antagonist effects throughout the body, counteracting effects such as [[itching]] and constipation, but without affecting opioid effects in the brain such as analgesia. | ||
| Line 38: | Line 41: | ||
==References== | ==References== | ||
{{Reflist|2}} | |||
* {{cite journal | author=Holzer P | title=Treatment of opioid-induced gut dysfunction | journal=Expert Opin Investig Drugs | year=2007 | pages=181-94 | volume=16 | issue=2 | id=PMID 17243938}} | * {{cite journal | author=Holzer P | title=Treatment of opioid-induced gut dysfunction | journal=Expert Opin Investig Drugs | year=2007 | pages=181-94 | volume=16 | issue=2 | id=PMID 17243938}} | ||
Latest revision as of 18:38, 15 April 2015
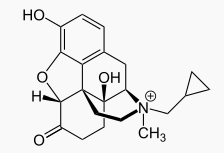 | |
| Clinical data | |
|---|---|
| Synonyms | Methylnaltrexone |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C21H26NO4 |
| Molar mass | 356.44 g/mol |
|
WikiDoc Resources for Methylnaltrexone |
|
Articles |
|---|
|
Most recent articles on Methylnaltrexone Most cited articles on Methylnaltrexone |
|
Media |
|
Powerpoint slides on Methylnaltrexone |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Methylnaltrexone at Clinical Trials.gov Trial results on Methylnaltrexone Clinical Trials on Methylnaltrexone at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Methylnaltrexone NICE Guidance on Methylnaltrexone
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Methylnaltrexone Discussion groups on Methylnaltrexone Patient Handouts on Methylnaltrexone Directions to Hospitals Treating Methylnaltrexone Risk calculators and risk factors for Methylnaltrexone
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Methylnaltrexone |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Methylnaltrexone (MTNX) is one of the newer agents of peripherally-acting μ-opioid antagonists that act to reverse some of the side effects of opioid drugs such as constipation without affecting analgesia or precipitating withdrawals. Because it contains a permanently charged tetravalent nitrogen atom, it cannot cross the blood-brain barrier, and so has antagonist effects throughout the body, counteracting effects such as itching and constipation, but without affecting opioid effects in the brain such as analgesia.
Development
In December of 2005, Wyeth and Progenics entered into an exclusive, worldwide agreement for the joint development and commercialization of methylnaltrexone for the treatment of opioid-induced side effects, including constipation and post-operative ileus (POI), a prolonged dysfunction of the gastrointestinal (GI) tract following surgery. Under the terms of the agreement, the companies are collaborating on worldwide development. Wyeth received worldwide rights to commercialize methylnaltrexone, and Progenics retained an option to co-promote the product in the United States. Wyeth will pay Progenics royalties on worldwide sales and co-promotion fees within the United States.
Methylnaltrexone is being developed in subcutaneous and oral forms to treat opioid induced constipation (OIC) and an intravenous form for POI.
Progenics and Wyeth are conducting two global phase 3 clinical trials in POI, targeting an NDA submission in this indication in early 2008. An oral formulation for OIC in patients with chronic pain currently is under development with an anticipated NDA submission in late 2009 or early 2010.
References
- Holzer P (2007). "Treatment of opioid-induced gut dysfunction". Expert Opin Investig Drugs. 16 (2): 181–94. PMID 17243938.
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Opioids
- Opioid antagonists
- Drugs