Sulfasalazine: Difference between revisions
No edit summary |
No edit summary |
||
| Line 63: | Line 63: | ||
*If such signs or symptoms are present, the patient should be evaluated immediately. | *If such signs or symptoms are present, the patient should be evaluated immediately. | ||
*Sulfasalazine should be discontinued if an alternative etiology for the signs or symptoms cannot be established. | *Sulfasalazine should be discontinued if an alternative etiology for the signs or symptoms cannot be established. | ||
|drugInteractions=*Reduced absorption of folic acid and digoxin have been reported when those agents were administered concomitantly with sulfasalazine. | |drugInteractions=*Reduced absorption of [[folic acid]] and [[digoxin]] have been reported when those agents were administered concomitantly with sulfasalazine. | ||
*When daily doses of sulfasalazine 2 g and weekly doses of methotrexate 7.5 mg were coadministered to 15 rheumatoid arthritis patients in a drug-drug interaction study, the pharmacokinetic disposition of the drugs was not altered. | *When daily doses of sulfasalazine 2 g and weekly doses of [[methotrexate]] 7.5 mg were coadministered to 15 [[rheumatoid arthritis]] patients in a drug-drug interaction study, the [[pharmacokinetic]] disposition of the drugs was not altered. | ||
*Daily doses of sulfasalazine 2 g (maximum 3 g) and weekly doses of methotrexate 7.5 mg (maximum 15 mg) were administered alone or in combination to 310 rheumatoid arthritis patients in two controlled 52-week clinical studies. | *Daily doses of sulfasalazine 2 g (maximum 3 g) and weekly doses of [[methotrexate]] 7.5 mg (maximum 15 mg) were administered alone or in combination to 310 [[rheumatoid arthritis]] patients in two controlled 52-week clinical studies. | ||
*The overall toxicity profile of the combination revealed an increased incidence of gastrointestinal adverse events, especially nausea, when compared to the incidence associated with either drug administered alone. | *The overall toxicity profile of the combination revealed an increased incidence of [[gastrointestinal]] adverse events, especially [[nausea]], when compared to the incidence associated with either drug administered alone. | ||
|FDAPregCat=B | |||
|useInPregnancyFDA=*There are no adequate and well-controlled studies of sulfasalazine in pregnant women. Reproduction studies have been performed in rats and rabbits at doses up to 6 times the human maintenance dose of 2 g/day based on body surface area and have revealed no evidence of impaired female fertility or harm to the fetus due to sulfasalazine. | |||
*Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | |||
*There have been case reports of neural tube defects (NTDs) in infants born to mothers who were exposed to sulfasalazine during pregnancy, but the role of sulfasalazine in these defects has not been established. | |||
*However, oral sulfasalazine inhibits the absorption and metabolism of [[folic acid]] which may interfere with folic acid supplementation and diminish the effect of periconceptional [[folic acid]] supplementation that has been shown to decrease the risk of NTDs. | |||
*A national survey evaluated the outcome of pregnancies associated with [[inflammatory bowel disease]] (IBD). In 186 pregnancies in women treated with sulfasalazine alone or sulfasalazine and concomitant steroid therapy, the incidence of fetal morbidity and mortality was comparable both to that of 245 untreated IBD pregnancies, and to pregnancies in the general population. | |||
*A study of 1455 pregnancies associated with exposure to [[sulfonamides]] including sulfasalazine, indicated that this group of drugs did not appear to be associated with fetal malformation. A review of the medical literature covering 1155 pregnancies in women with [[ulcerative colitis]] suggested that the outcome was similar to that expected in the general population. | |||
*No clinical studies have been performed to evaluate the effect of sulfasalazine on the growth development and functional maturation of children whose mothers received the drug during pregnancy. | |||
*Sulfasalazine and its metabolite, sulfapyridine, pass through the [[placenta]]. | |||
*Sulfasalazine and its metabolite are also present in human milk. | |||
*In the newborn, [[sulfonamides]] compete with [[bilirubin]] for binding sites on the plasma proteins and may cause [[kernicterus]]. Although sulfapyridine has been shown to have poor bilirubin-displacing capacity, monitor the newborn for the potential for [[kernicterus]]. | |||
*A case of [[agranulocytosis]] has been reported in an infant whose mother was taking both sulfasalazine and prednisone throughout pregnancy. | |||
|AUSPregCat=A | |||
|useInNursing=*[[Sulfonamides]], including sulfasalazine, are present in human milk. *Insignificant amounts of sulfasalazine have been found in milk, whereas levels of the active metabolite sulfapyridine in milk are about 30 to 60 percent of those in the maternal serum. | |||
*Caution should be exercised when AZULFIDINE EN-tabs is administered to a nursing mother. | |||
*There are reports with limited data of bloody stools or [[diarrhea]] in human milk fed infants of mothers taking sulfasalazine. In cases where the outcome was reported, [[bloody stools]] or [[diarrhea]] resolved in the infant after discontinuation of sulfasalazine in the mother or discontinuation of breastfeeding. Due to limited data, a causal relationship between sulfasalazine exposure and [[bloody stools]] or [[diarrhea]] cannot be confirmed or denied. Monitor human milk fed infants of mothers taking sulfasalazine for signs and symptoms of [[diarrhea]] and/or [[bloody stools]]. | |||
|useInPed=The safety and effectiveness of AZULFIDINE EN-tabs in pediatric patients below the age of 2 years with ulcerative colitis have not been established. | |||
The safety and effectiveness of AZULFIDINE EN-tabs for the treatment of the signs and symptoms of polyarticular-course juvenile rheumatoid arthritis in pediatric patients aged 6–16 years is supported by evidence from adequate and well-controlled studies in adult rheumatoid arthritis patients. The extrapolation from adults with rheumatoid arthritis to children with polyarticular-course juvenile rheumatoid arthritis is based on similarities in disease and response to therapy between these two patient populations. Published studies support the extrapolation of safety and effectiveness for sulfasalazine to polyarticular-course juvenile rheumatoid arthritis1,5 (see ADVERSE REACTIONS). | |||
It has been reported that the frequency of adverse events in patients with systemic-course of juvenile arthritis is high.6 Use in children with systemic-course juvenile rheumatoid arthritis has frequently resulted in a serum sickness-like reaction.5 This reaction is often severe and presents as fever, nausea, vomiting, headache, rash, and abnormal liver function tests. Treatment of systemic-course juvenile rheumatoid arthritis with sulfasalazine is not recommended. | |||
*Small studies have been reported in the literature in children down to the age of 4 years with ulcerative colitis and inflammatory bowel disease. | |||
*In these populations, relative to adults, the pharmacokinetics of SSZ and SP correlated poorly with either age or dose. | |||
*To date, comparative pharmacokinetic trials have not been conducted to determine whether or not significant pharmacokinetic differences exist between children with juvenile rheumatoid arthritis and adults with rheumatoid arthritis. | |||
|useInGeri=*Elderly patients with rheumatoid arthritis showed a prolonged plasma half-life for SSZ, SP, and their metabolites. The clinical impact of this is unknown. | |||
|useInGender=*Gender appears not to have an effect on either the rate or the pattern of metabolites of SSZ, SP, or 5-ASA. | |||
|useInRace=The metabolism of SP to AcSP is mediated by polymorphic enzymes such that two distinct populations of slow and fast metabolizers exist. Approximately 60% of the Caucasian population can be classified as belonging to the slow acetylator phenotype. These subjects will display a prolonged plasma half-life for SP (14.8 hrs vs. 10.4 hrs) and an accumulation of higher plasma levels of SP than fast acetylators. The clinical implication of this is unclear; however, in a small pharmacokinetic trial where acetylator status was determined, subjects who were slow acetylators of SP showed a higher incidence of adverse events. | |||
|administration=*Oral | |administration=*Oral | ||
|monitoring=*Complete blood counts, as well as urinalysis with careful microscopic examination, should be done frequently in patients receiving AZULFIDINE EN-tabs. | |monitoring=*Complete blood counts, as well as urinalysis with careful microscopic examination, should be done frequently in patients receiving AZULFIDINE EN-tabs. | ||
*Closely monitor patients for the development of signs and symptoms of infection during and after treatment with AZULFIDINE EN-tabs. | *Closely monitor patients for the development of signs and symptoms of infection during and after treatment with AZULFIDINE EN-tabs. | ||
|overdose=There is evidence that the incidence and severity of toxicity following overdosage is directly related to the total serum sulfapyridine concentration. Symptoms of overdosage may include [[nausea]], [[vomiting]], gastric distress and abdominal pains. In more advanced cases, central nervous system symptoms such as [[drowsiness]], [[convulsions]], etc., may be observed. Serum sulfapyridine concentrations may be used to monitor the progress of recovery from overdosage. | |||
*There are no documented reports of deaths due to ingestion of large single doses of sulfasalazine. It has not been possible to determine the LD50 in laboratory animals such as mice, since the highest oral daily dose of sulfasalazine which can be given (12 g/kg) is not lethal. Doses of regular sulfasalazine tablets of 16 g per day have been given to patients without mortality. | |||
====Instructions for Overdosage==== | |||
[[Gastric lavage]] or [[emesis]] plus [[catharsis]] as indicated. Alkalinize [[urine]]. If [[kidney function]] is normal, force fluids. If [[anuria]] is present, restrict fluids and salt, and treat appropriately. Catheterization of the ureters may be indicated for complete renal blockage by crystals. The low molecular weight of sulfasalazine and its metabolites may facilitate their removal by [[dialysis]]. | |||
|drugBox={{Drugbox | |||
| Verifiedfields = changed | |||
| verifiedrevid = 460764435 | |||
| IUPAC_name = 2-hydroxy-5-[(''E'')-2-{4-[(pyridin-2-yl)sulfamoyl]phenyl}diazen-1-yl]benzoic acid | |||
| image = [[File:Sulfasalazine chemical structure.png|none|27px]] | |||
<!--Clinical data--> | |||
| tradename = Azulfidine | |||
| Drugs.com = {{drugs.com|monograph|sulfasalazine}} | |||
| MedlinePlus = a682204 | |||
| pregnancy_category = b <ref>http://www.drugs.com/pregnancy/sulfasalazine.html</ref> | |||
| legal_status = | |||
| routes_of_administration = oral | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = <15%<references/> | |||
| metabolism = | |||
| elimination_half-life = 5-10 hours | |||
| excretion = | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 599-79-1 | |||
| ATC_prefix = A07 | |||
| ATC_suffix = EC01 | |||
| PubChem = 5384001 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00795 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 10481900 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 3XC8GUZ6CB | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00448 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 421 | |||
<!--Chemical data--> | |||
| C=18 | H=14 | N=4 | O=5 | S=1 | |||
| molecular_weight = 398.394 g/mol | |||
| smiles = O=S(=O)(Nc1ccccn1)c3ccc(/N=N/c2cc(C(O)=O)c(O)cc2)cc3 | |||
| InChI = 1/C18H14N4O5S/c23-16-9-6-13(11-15(16)18(24)25)21-20-12-4-7-14(8-5-12)28(26,27)22-17-3-1-2-10-19-17/h1-11,23H,(H,19,22)(H,24,25) | |||
| InChIKey = NCEXYHBECQHGNR-UHFFFAOYAO | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C18H14N4O5S/c23-16-9-6-13(11-15(16)18(24)25)21-20-12-4-7-14(8-5-12)28(26,27)22-17-3-1-2-10-19-17/h1-11,23H,(H,19,22)(H,24,25) | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = NCEXYHBECQHGNR-UHFFFAOYSA-N | |||
}} | |||
|structure=*Chemical Designation: 5-([p-(2-pyridylsulfamoyl)phenyl]azo) salicylic acid. | |||
[[File:Sulfasalazine chemical.png|none|400px]] | |||
|PD=*The mode of action of sulfasalazine (SSZ) or its metabolites, 5-aminosalicylic acid (5-ASA) and sulfapyridine (SP), is still under investigation, but may be related to the [[anti-inflammatory]] and/or immunomodulatory properties that have been observed in animal and [[in vitro]] models, to its affinity for [[connective tissue]], and/or to the relatively high concentration it reaches in [[serous fluids]], the [[liver]] and intestinal walls, as demonstrated in autoradiographic studies in animals. | |||
*In [[ulcerative colitis]], clinical studies utilizing rectal administration of SSZ, SP and 5-ASA have indicated that the major therapeutic action may reside in the 5-ASA moiety. | |||
*The relative contribution of the parent drug and the major metabolites in [[rheumatoid arthritis]] is unknown. | |||
|PK=*[[In vivo]] studies have indicated that the absolute [[bioavailability]] of orally administered SSZ is less than 15% for parent drug. | |||
*In the [[intestine]], SSZ is metabolized by [[intestinal bacteria]] to SP and 5-ASA. | |||
*Of the two species, SP is relatively well absorbed from the [[intestine]] and highly metabolized, while 5-ASA is much less well absorbed. | |||
====Absorption==== | |||
*Following oral administration of 1 g of SSZ to 9 healthy males, less than 15% of a dose of SSZ is absorbed as parent drug. | |||
*Detectable serum concentrations of SSZ have been found in healthy subjects within 90 minutes after the ingestion. | |||
*Maximum concentrations of SSZ occur between 3 and 12 hours post-ingestion, with the mean peak concentration (6 µg/mL) occurring at 6 hours. | |||
*In comparison, peak plasma levels of both SP and 5-ASA occur approximately 10 hours after dosing. | |||
*This longer time to peak is indicative of gastrointestinal transit to the lower [[intestine]], where bacteria-mediated metabolism occurs. SP apparently is well absorbed from the [[colon]], with an estimated bioavailability of 60%. In this same study, 5-ASA is much less well absorbed from the [[gastrointestinal tract]], with an estimated [[bioavailability]] of from 10% to 30%. | |||
====Distribution==== | |||
*Following intravenous injection, the calculated volume of distribution (Vdss) for SSZ was 7.5 ± 1.6 L. | |||
*SSZ is highly bound to albumin (>99.3%), while SP is only about 70% bound to albumin. | |||
Acetylsulfapyridine (AcSP), the principal metabolite of SP, is approximately 90% bound to plasma proteins. | |||
====Metabolism==== | |||
*As mentioned above, SSZ is metabolized by intestinal bacteria to SP and 5-ASA. *Approximately 15% of a dose of SSZ is absorbed as parent and is metabolized to some extent in the liver to the same two species. | |||
*The observed plasma half-life for intravenous sulfasalazine is 7.6 ± 3.4 hrs. *The primary route of metabolism of SP is via acetylation to form AcSP. | |||
*The rate of metabolism of SP to AcSP is dependent upon acetylator phenotype. *In fast acetylators, the mean plasma half-life of SP is 10.4 hrs, while in slow acetylators it is 14.8 hrs. | |||
*SP can also be metabolized to 5-hydroxy-sulfapyridine (SPOH) and N-acetyl-5-hydroxy-sulfapyridine. | |||
*5-ASA is primarily metabolized in both the liver and intestine to N-acetyl-5-aminosalicylic acid via a nonacetylation phenotype dependent route. | |||
*Due to low plasma levels produced by 5-ASA after oral administration, reliable estimates of plasma half-life are not possible. | |||
====Excretion==== | |||
*Absorbed SP and 5-ASA and their metabolites are primarily eliminated in the urine either as free metabolites or as glucuronide conjugates. | |||
*The majority of 5-ASA stays within the colonic lumen and is excreted as 5-ASA and acetyl-5-ASA with the feces. | |||
*The calculated clearance of SSZ following intravenous administration was 1 L/hr. | |||
*[[Renal clearance]] was estimated to account for 37% of total clearance. | |||
|howSupplied=*AZULFIDINE EN-tabs Tablets, 500 mg, are elliptical, gold-colored, film enteric-coated tablets, monogrammed "102" on one side and "KPh" on the other. They are available in the following package sizes: | |||
**Bottles of 100 NDC 0013-0102-01 | |||
**Bottles of 300 NDC 0013-0102-20 | |||
|storage=*Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) | |||
|packLabel=[[File:Sulfasalazinr FDA label.png|none|400px]] | |||
|fdaPatientInfo=*Patients should be informed of the possibility of adverse effects and of the need for careful medical supervision. | |||
*The occurrence of [[sore throat]], [[fever]], [[pallor]], [[purpura]] or [[jaundice]] may indicate a serious blood disorder. Should any of these occur, the patient should seek medical advice. | |||
*Patients should be instructed to take AZULFIDINE EN-tabs in evenly divided doses, preferably after meals, and to swallow the tablets whole. | |||
Additionally, patients should be advised that sulfasalazine may produce an orange-yellow discoloration of the [[urine]] or [[skin]]. | |||
|alcohol=Alcohol-Sulfasalazine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Sulfasalazine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=*Azulfidine | |||
*Azulfidine Entabs | |||
*Sulfazine | |||
*Sulfazine EC | |||
}} | |||
{{LabelImage | |||
|fileName=Sulfasalazine package wikidoc.png | |||
}} | }} | ||
{{LabelImage}} | |||
Revision as of 20:35, 9 January 2015
{{DrugProjectFormSinglePage |authorTag=Stefano Giannoni [1] |genericName=Sulfasalazine |aOrAn=a |drugClass=Sulfonamide |indicationType=treatment |indication=mild to moderate ulcerative colitis, as adjunctive therapy in severe ulcerative colitis, for the prolongation of the remission period between acute attacks of ulcerative colitis, rheumatoid arthritis and for pediatric patients with polyarticular-course juvenile rheumatoid arthritis. |blackBoxWarningTitle=TITLE |blackBoxWarningBody=Condition Name: (Content) |fdaLIADAdult=====Ulcerative Colitis==== The dosage of AZULFIDINE EN-tabs Tablets should be adjusted to each individual's response and tolerance. Patients should be instructed to take AZULFIDINE EN-tabs in evenly divided doses, preferably after meals, and to swallow the tablets whole.
Initial Therapy
- 3 to 4 g daily in evenly divided doses with dosage intervals not exceeding eight hours.
- It may be advisable to initiate therapy with a lower dosage, e.g., 1 to 2 g daily, to reduce possible gastrointestinal intolerance.
- If daily doses exceeding 4 g are required to achieve the desired therapeutic effect, the increased risk of toxicity should be kept in mind.
Maintenance Therapy
- 2 g daily
Rheumatoid Arthritis
- 2 g daily in two evenly divided doses.
- It is advisable to initiate therapy with a lower dosage of AZULFIDINE EN-tabs, e.g., 0.5 to 1.0 g daily, to reduce possible gastrointestinal intolerance.
- Careful monitoring is recommended for doses over 2 g per day.
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Sulfasalazine in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Sulfasalazine in adult patients. |fdaLIADPed=====Ulcerative Colitis==== The dosage of AZULFIDINE EN-tabs Tablets should be adjusted to each individual's response and tolerance. Patients should be instructed to take AZULFIDINE EN-tabs in evenly divided doses, preferably after meals, and to swallow the tablets whole.
Initial Therapy
- 40 to 60 mg/kg of body weight in each 24-hour period, divided into 3 to 6 doses.
- Six years of age and older
Maintenance Therapy
- 30 mg/kg of body weight in each 24-hour period, divided into 4 doses.
- Six years of age and older
Juvenile Rheumatoid Arthritis, Polyarticular-Course
- 30 to 50 mg/kg of body weight daily in two evenly divided doses.
- Maximum dose is 2 g per day.
- To reduce possible gastrointestinal intolerance, begin with a quarter to a third of the planned maintenance dose and increase weekly until reaching the maintenance dose at one month.
- Six years of age and older
|offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Sulfasalazine in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Sulfasalazine in pediatric patients. |contraindications=*Hypersensitivity to sulfasalazine, its metabolites, sulfonamides or salicylates.
- Patients with intestinal or urinary obstruction,
- Patients with porphyria, as the sulfonamides have been reported to precipitate an acute attack.
|warnings=*Only after critical appraisal should AZULFIDINE EN-tabs Tablets be given to patients with hepatic or renal damage or blood dyscrasias.
- Deaths associated with the administration of sulfasalazine have been reported from hypersensitivity reactions, agranulocytosis, aplastic anemia, other blood dyscrasias, renal and liver damage, irreversible neuromuscular and central nervous system changes, and fibrosing alveolitis.
- The presence of clinical signs such as sore throat, fever, pallor, purpura or jaundice may be indications of serious blood disorders or hepatotoxicity.
- Complete blood counts, as well as urinalysis with careful microscopic examination, should be done frequently in patients receiving AZULFIDINE EN-tabs.
- Discontinue treatment with sulfasalazine while awaiting the results of blood tests.
- Oligospermia and infertility have been observed in men treated with sulfasalazine; however, withdrawal of the drug appears to reverse these effects.
- Serious infections, including fatal sepsis and pneumonia, have been reported. Some infections were associated with agranulocytosis, neutropenia, or myelosuppression.
- Discontinue AZULFIDINE EN-tabs if a patient develops a serious infection. *Closely monitor patients for the development of signs and symptoms of infection during and after treatment with AZULFIDINE EN-tabs.
- For a patient who develops a new infection during treatment with AZULFIDINE EN-tabs, perform a prompt and complete diagnostic workup for infection and myelosuppression.
- Caution should be exercised when considering the use of sulfasalazine in patients with a history of recurring or chronic infections or with underlying conditions or concomitant drugs which may predispose patients to infections.
- Severe hypersensitivity reactions may include internal organ involvement, such as hepatitis, nephritis, myocarditis, mononucleosis-like syndrome (i.e., pseudomononucleosis), hematological abnormalities (including hematophagic histiocytosis), and/or pneumonitis including eosinophilic infiltration.
- Serious skin reactions, some of them fatal, including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, have been reported in association with the use of sulfasalazine.
- Patients are at highest risk for these events early in therapy, with most events occurring within the first month of treatment.
- Sulfasalazine should be discontinued at the first appearance of skin rash, mucosal lesions, or any other sign of hypersensitivity.
- Severe, life-threatening, systemic hypersensitivity reactions such as drug rash with eosinophilia and systemic symptoms have been reported in patients taking sulfasalazine.
- Early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident.
- If such signs or symptoms are present, the patient should be evaluated immediately.
- Sulfasalazine should be discontinued if an alternative etiology for the signs or symptoms cannot be established.
|drugInteractions=*Reduced absorption of folic acid and digoxin have been reported when those agents were administered concomitantly with sulfasalazine.
- When daily doses of sulfasalazine 2 g and weekly doses of methotrexate 7.5 mg were coadministered to 15 rheumatoid arthritis patients in a drug-drug interaction study, the pharmacokinetic disposition of the drugs was not altered.
- Daily doses of sulfasalazine 2 g (maximum 3 g) and weekly doses of methotrexate 7.5 mg (maximum 15 mg) were administered alone or in combination to 310 rheumatoid arthritis patients in two controlled 52-week clinical studies.
- The overall toxicity profile of the combination revealed an increased incidence of gastrointestinal adverse events, especially nausea, when compared to the incidence associated with either drug administered alone.
|FDAPregCat=B |useInPregnancyFDA=*There are no adequate and well-controlled studies of sulfasalazine in pregnant women. Reproduction studies have been performed in rats and rabbits at doses up to 6 times the human maintenance dose of 2 g/day based on body surface area and have revealed no evidence of impaired female fertility or harm to the fetus due to sulfasalazine.
- Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- There have been case reports of neural tube defects (NTDs) in infants born to mothers who were exposed to sulfasalazine during pregnancy, but the role of sulfasalazine in these defects has not been established.
- However, oral sulfasalazine inhibits the absorption and metabolism of folic acid which may interfere with folic acid supplementation and diminish the effect of periconceptional folic acid supplementation that has been shown to decrease the risk of NTDs.
- A national survey evaluated the outcome of pregnancies associated with inflammatory bowel disease (IBD). In 186 pregnancies in women treated with sulfasalazine alone or sulfasalazine and concomitant steroid therapy, the incidence of fetal morbidity and mortality was comparable both to that of 245 untreated IBD pregnancies, and to pregnancies in the general population.
- A study of 1455 pregnancies associated with exposure to sulfonamides including sulfasalazine, indicated that this group of drugs did not appear to be associated with fetal malformation. A review of the medical literature covering 1155 pregnancies in women with ulcerative colitis suggested that the outcome was similar to that expected in the general population.
- No clinical studies have been performed to evaluate the effect of sulfasalazine on the growth development and functional maturation of children whose mothers received the drug during pregnancy.
- Sulfasalazine and its metabolite, sulfapyridine, pass through the placenta.
- Sulfasalazine and its metabolite are also present in human milk.
- In the newborn, sulfonamides compete with bilirubin for binding sites on the plasma proteins and may cause kernicterus. Although sulfapyridine has been shown to have poor bilirubin-displacing capacity, monitor the newborn for the potential for kernicterus.
- A case of agranulocytosis has been reported in an infant whose mother was taking both sulfasalazine and prednisone throughout pregnancy.
|AUSPregCat=A |useInNursing=*Sulfonamides, including sulfasalazine, are present in human milk. *Insignificant amounts of sulfasalazine have been found in milk, whereas levels of the active metabolite sulfapyridine in milk are about 30 to 60 percent of those in the maternal serum.
- Caution should be exercised when AZULFIDINE EN-tabs is administered to a nursing mother.
- There are reports with limited data of bloody stools or diarrhea in human milk fed infants of mothers taking sulfasalazine. In cases where the outcome was reported, bloody stools or diarrhea resolved in the infant after discontinuation of sulfasalazine in the mother or discontinuation of breastfeeding. Due to limited data, a causal relationship between sulfasalazine exposure and bloody stools or diarrhea cannot be confirmed or denied. Monitor human milk fed infants of mothers taking sulfasalazine for signs and symptoms of diarrhea and/or bloody stools.
|useInPed=The safety and effectiveness of AZULFIDINE EN-tabs in pediatric patients below the age of 2 years with ulcerative colitis have not been established.
The safety and effectiveness of AZULFIDINE EN-tabs for the treatment of the signs and symptoms of polyarticular-course juvenile rheumatoid arthritis in pediatric patients aged 6–16 years is supported by evidence from adequate and well-controlled studies in adult rheumatoid arthritis patients. The extrapolation from adults with rheumatoid arthritis to children with polyarticular-course juvenile rheumatoid arthritis is based on similarities in disease and response to therapy between these two patient populations. Published studies support the extrapolation of safety and effectiveness for sulfasalazine to polyarticular-course juvenile rheumatoid arthritis1,5 (see ADVERSE REACTIONS).
It has been reported that the frequency of adverse events in patients with systemic-course of juvenile arthritis is high.6 Use in children with systemic-course juvenile rheumatoid arthritis has frequently resulted in a serum sickness-like reaction.5 This reaction is often severe and presents as fever, nausea, vomiting, headache, rash, and abnormal liver function tests. Treatment of systemic-course juvenile rheumatoid arthritis with sulfasalazine is not recommended.
- Small studies have been reported in the literature in children down to the age of 4 years with ulcerative colitis and inflammatory bowel disease.
- In these populations, relative to adults, the pharmacokinetics of SSZ and SP correlated poorly with either age or dose.
- To date, comparative pharmacokinetic trials have not been conducted to determine whether or not significant pharmacokinetic differences exist between children with juvenile rheumatoid arthritis and adults with rheumatoid arthritis.
|useInGeri=*Elderly patients with rheumatoid arthritis showed a prolonged plasma half-life for SSZ, SP, and their metabolites. The clinical impact of this is unknown. |useInGender=*Gender appears not to have an effect on either the rate or the pattern of metabolites of SSZ, SP, or 5-ASA. |useInRace=The metabolism of SP to AcSP is mediated by polymorphic enzymes such that two distinct populations of slow and fast metabolizers exist. Approximately 60% of the Caucasian population can be classified as belonging to the slow acetylator phenotype. These subjects will display a prolonged plasma half-life for SP (14.8 hrs vs. 10.4 hrs) and an accumulation of higher plasma levels of SP than fast acetylators. The clinical implication of this is unclear; however, in a small pharmacokinetic trial where acetylator status was determined, subjects who were slow acetylators of SP showed a higher incidence of adverse events. |administration=*Oral |monitoring=*Complete blood counts, as well as urinalysis with careful microscopic examination, should be done frequently in patients receiving AZULFIDINE EN-tabs.
- Closely monitor patients for the development of signs and symptoms of infection during and after treatment with AZULFIDINE EN-tabs.
|overdose=There is evidence that the incidence and severity of toxicity following overdosage is directly related to the total serum sulfapyridine concentration. Symptoms of overdosage may include nausea, vomiting, gastric distress and abdominal pains. In more advanced cases, central nervous system symptoms such as drowsiness, convulsions, etc., may be observed. Serum sulfapyridine concentrations may be used to monitor the progress of recovery from overdosage.
- There are no documented reports of deaths due to ingestion of large single doses of sulfasalazine. It has not been possible to determine the LD50 in laboratory animals such as mice, since the highest oral daily dose of sulfasalazine which can be given (12 g/kg) is not lethal. Doses of regular sulfasalazine tablets of 16 g per day have been given to patients without mortality.
Instructions for Overdosage
Gastric lavage or emesis plus catharsis as indicated. Alkalinize urine. If kidney function is normal, force fluids. If anuria is present, restrict fluids and salt, and treat appropriately. Catheterization of the ureters may be indicated for complete renal blockage by crystals. The low molecular weight of sulfasalazine and its metabolites may facilitate their removal by dialysis. |drugBox={{Drugbox | Verifiedfields = changed | verifiedrevid = 460764435 | IUPAC_name = 2-hydroxy-5-[(E)-2-{4-[(pyridin-2-yl)sulfamoyl]phenyl}diazen-1-yl]benzoic acid
| image =
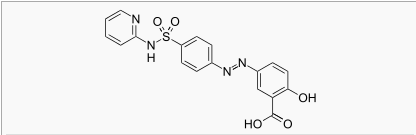
| tradename = Azulfidine | Drugs.com = Monograph | MedlinePlus = a682204 | pregnancy_category = b [1] | legal_status = | routes_of_administration = oral
| bioavailability = <15%
| metabolism = | elimination_half-life = 5-10 hours | excretion =
| CASNo_Ref =
| CAS_number_Ref =
| CAS_number = 599-79-1
| ATC_prefix = A07
| ATC_suffix = EC01
| PubChem = 5384001
| DrugBank_Ref =
| DrugBank = DB00795
| ChemSpiderID_Ref =
| ChemSpiderID = 10481900
| UNII_Ref =
| UNII = 3XC8GUZ6CB
| KEGG_Ref =
| KEGG = D00448
| ChEMBL_Ref =
| ChEMBL = 421
| C=18 | H=14 | N=4 | O=5 | S=1
| molecular_weight = 398.394 g/mol
| smiles = O=S(=O)(Nc1ccccn1)c3ccc(/N=N/c2cc(C(O)=O)c(O)cc2)cc3
| InChI = 1/C18H14N4O5S/c23-16-9-6-13(11-15(16)18(24)25)21-20-12-4-7-14(8-5-12)28(26,27)22-17-3-1-2-10-19-17/h1-11,23H,(H,19,22)(H,24,25)
| InChIKey = NCEXYHBECQHGNR-UHFFFAOYAO
| StdInChI_Ref =
| StdInChI = 1S/C18H14N4O5S/c23-16-9-6-13(11-15(16)18(24)25)21-20-12-4-7-14(8-5-12)28(26,27)22-17-3-1-2-10-19-17/h1-11,23H,(H,19,22)(H,24,25)
| StdInChIKey_Ref =
| StdInChIKey = NCEXYHBECQHGNR-UHFFFAOYSA-N
}}
|structure=*Chemical Designation: 5-([p-(2-pyridylsulfamoyl)phenyl]azo) salicylic acid.
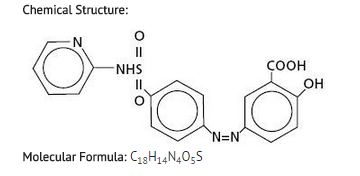
|PD=*The mode of action of sulfasalazine (SSZ) or its metabolites, 5-aminosalicylic acid (5-ASA) and sulfapyridine (SP), is still under investigation, but may be related to the anti-inflammatory and/or immunomodulatory properties that have been observed in animal and in vitro models, to its affinity for connective tissue, and/or to the relatively high concentration it reaches in serous fluids, the liver and intestinal walls, as demonstrated in autoradiographic studies in animals.
- In ulcerative colitis, clinical studies utilizing rectal administration of SSZ, SP and 5-ASA have indicated that the major therapeutic action may reside in the 5-ASA moiety.
- The relative contribution of the parent drug and the major metabolites in rheumatoid arthritis is unknown.
|PK=*In vivo studies have indicated that the absolute bioavailability of orally administered SSZ is less than 15% for parent drug.
- In the intestine, SSZ is metabolized by intestinal bacteria to SP and 5-ASA.
- Of the two species, SP is relatively well absorbed from the intestine and highly metabolized, while 5-ASA is much less well absorbed.
Absorption
- Following oral administration of 1 g of SSZ to 9 healthy males, less than 15% of a dose of SSZ is absorbed as parent drug.
- Detectable serum concentrations of SSZ have been found in healthy subjects within 90 minutes after the ingestion.
- Maximum concentrations of SSZ occur between 3 and 12 hours post-ingestion, with the mean peak concentration (6 µg/mL) occurring at 6 hours.
- In comparison, peak plasma levels of both SP and 5-ASA occur approximately 10 hours after dosing.
- This longer time to peak is indicative of gastrointestinal transit to the lower intestine, where bacteria-mediated metabolism occurs. SP apparently is well absorbed from the colon, with an estimated bioavailability of 60%. In this same study, 5-ASA is much less well absorbed from the gastrointestinal tract, with an estimated bioavailability of from 10% to 30%.
Distribution
- Following intravenous injection, the calculated volume of distribution (Vdss) for SSZ was 7.5 ± 1.6 L.
- SSZ is highly bound to albumin (>99.3%), while SP is only about 70% bound to albumin.
Acetylsulfapyridine (AcSP), the principal metabolite of SP, is approximately 90% bound to plasma proteins.
Metabolism
- As mentioned above, SSZ is metabolized by intestinal bacteria to SP and 5-ASA. *Approximately 15% of a dose of SSZ is absorbed as parent and is metabolized to some extent in the liver to the same two species.
- The observed plasma half-life for intravenous sulfasalazine is 7.6 ± 3.4 hrs. *The primary route of metabolism of SP is via acetylation to form AcSP.
- The rate of metabolism of SP to AcSP is dependent upon acetylator phenotype. *In fast acetylators, the mean plasma half-life of SP is 10.4 hrs, while in slow acetylators it is 14.8 hrs.
- SP can also be metabolized to 5-hydroxy-sulfapyridine (SPOH) and N-acetyl-5-hydroxy-sulfapyridine.
- 5-ASA is primarily metabolized in both the liver and intestine to N-acetyl-5-aminosalicylic acid via a nonacetylation phenotype dependent route.
- Due to low plasma levels produced by 5-ASA after oral administration, reliable estimates of plasma half-life are not possible.
Excretion
- Absorbed SP and 5-ASA and their metabolites are primarily eliminated in the urine either as free metabolites or as glucuronide conjugates.
- The majority of 5-ASA stays within the colonic lumen and is excreted as 5-ASA and acetyl-5-ASA with the feces.
- The calculated clearance of SSZ following intravenous administration was 1 L/hr.
- Renal clearance was estimated to account for 37% of total clearance.
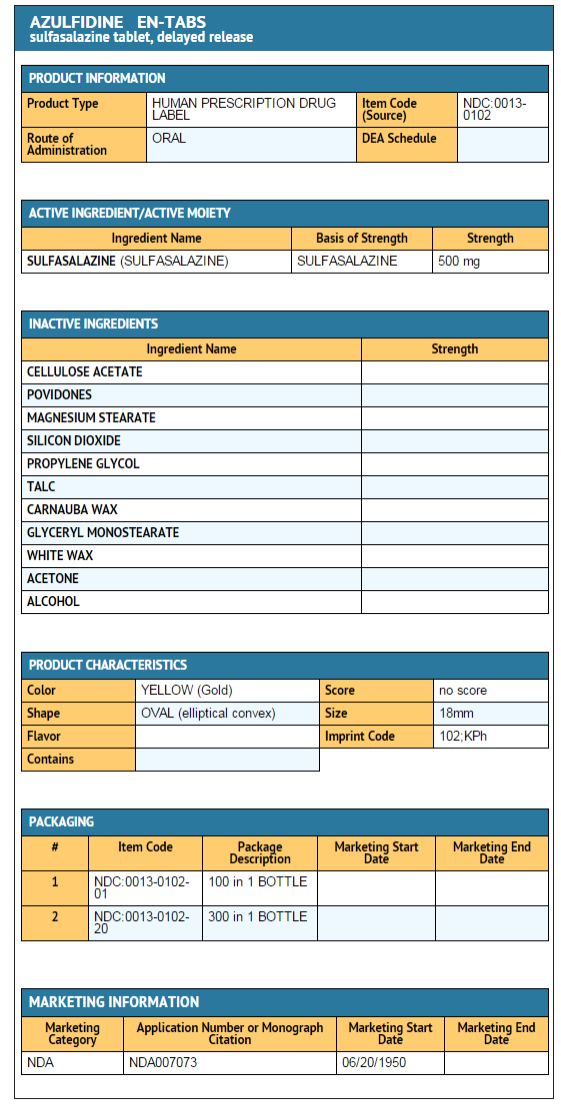
|howSupplied=*AZULFIDINE EN-tabs Tablets, 500 mg, are elliptical, gold-colored, film enteric-coated tablets, monogrammed "102" on one side and "KPh" on the other. They are available in the following package sizes:
- Bottles of 100 NDC 0013-0102-01
- Bottles of 300 NDC 0013-0102-20
|storage=*Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F)
|packLabel=
|fdaPatientInfo=*Patients should be informed of the possibility of adverse effects and of the need for careful medical supervision.
- The occurrence of sore throat, fever, pallor, purpura or jaundice may indicate a serious blood disorder. Should any of these occur, the patient should seek medical advice.
- Patients should be instructed to take AZULFIDINE EN-tabs in evenly divided doses, preferably after meals, and to swallow the tablets whole.
Additionally, patients should be advised that sulfasalazine may produce an orange-yellow discoloration of the urine or skin. |alcohol=Alcohol-Sulfasalazine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. |brandNames=*Azulfidine
- Azulfidine Entabs
- Sulfazine
- Sulfazine EC
}} {{#subobject:
|Label Page=Sulfasalazine |Label Name=Sulfasalazine package wikidoc.png
}}
{{#subobject:
|Label Page=Sulfasalazine |Label Name=
}}