RELYVRIO: Difference between revisions
No edit summary |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 12: | Line 12: | ||
RELYVRIO has a high sodium content. In patients sensitive to salt intake, consider the amount of daily sodium intake in each dose of RELYVRIO and monitor appropriately. | RELYVRIO has a high sodium content. In patients sensitive to salt intake, consider the amount of daily sodium intake in each dose of RELYVRIO and monitor appropriately. | ||
|fdaLIADAdult=The recommended dosage is 1 packet (3 g sodium phenylbutyrate and 1 g taurursodiol) administered orally or via feeding tube as follows: | |fdaLIADAdult=The recommended dosage is 1 packet (3 g sodium phenylbutyrate and 1 g taurursodiol) administered orally or via feeding tube as follows: | ||
Initial dosage: 1 packet daily for the first 3 weeks | *Initial dosage: 1 packet daily for the first 3 weeks | ||
maintenance dosage: 1 packet twice daily thereafter | *maintenance dosage: 1 packet twice daily thereafter | ||
Empty contents of one packet in a cup containing 8 ounces of room temperature water and stir vigorously prior to administration. | *Empty contents of one packet in a cup containing 8 ounces of room temperature water and stir vigorously prior to administration. | ||
Take within 1 hour of preparation. | *Take within 1 hour of preparation. | ||
Administer RELYVRIO before a snack or meal. | *Administer RELYVRIO before a snack or meal. | ||
|fdaLIADPed=The safety and efficacy of RELYVRIO in pediatric patients have not been confirmed or established. | |fdaLIADPed=The safety and efficacy of RELYVRIO in pediatric patients have not been confirmed or established. | ||
|contraindications=None. | |contraindications=None. | ||
| Line 28: | Line 28: | ||
|clinicalTrials=In Study 1, involving 137 adult ALS patients randomized to either RELYVRIO or placebo for 24 weeks, safety was assessed. During this period, 6% of RELYVRIO-treated patients and 4% of placebo patients died, with the deaths attributed to ALS disease progression. The most common adverse reactions associated with RELYVRIO, occurring in at least 15% and at least 5% more than placebo, included diarrhea, abdominal pain, nausea, and upper respiratory tract infection. Gastrointestinal-related adverse reactions were more frequent in the first 3 weeks of treatment. Table 1 summarizes adverse reactions reported in over 5% of ALS patients taking RELYVRIO and at least 5% greater than those taking placebo in Study 1, with diarrhea and abdominal pain being the most prevalent. | |clinicalTrials=In Study 1, involving 137 adult ALS patients randomized to either RELYVRIO or placebo for 24 weeks, safety was assessed. During this period, 6% of RELYVRIO-treated patients and 4% of placebo patients died, with the deaths attributed to ALS disease progression. The most common adverse reactions associated with RELYVRIO, occurring in at least 15% and at least 5% more than placebo, included diarrhea, abdominal pain, nausea, and upper respiratory tract infection. Gastrointestinal-related adverse reactions were more frequent in the first 3 weeks of treatment. Table 1 summarizes adverse reactions reported in over 5% of ALS patients taking RELYVRIO and at least 5% greater than those taking placebo in Study 1, with diarrhea and abdominal pain being the most prevalent. | ||
|drugInteractions=Potential for Other Drugs to Affect RELYVRIO: | |drugInteractions=Potential for Other Drugs to Affect RELYVRIO: | ||
*Bile Acid Sequestering Agents and Inhibitors of Bile Acid Transporters: | |||
Bile acid sequestering agents (e.g., [[cholestyramine]], [[colestipol]], colesevelam) may interfere with the absorption of bile acids such as taurursodiol. Avoid use of bile acid sequestering agents with RELYVRIO and consider alternative cholesterol lowering agents. | Bile acid sequestering agents (e.g., [[cholestyramine]], [[colestipol]], colesevelam) may interfere with the absorption of bile acids such as taurursodiol. Avoid use of bile acid sequestering agents with RELYVRIO and consider alternative cholesterol lowering agents. | ||
| Line 34: | Line 34: | ||
Avoid use of strong inhibitors of the bile salt export pump (BSEP) with RELYVRIO. If simultaneous use of a strong BSEP inhibitor like [[cyclosporine]] is deemed essential, exercise caution and monitor serum transaminases and bilirubin levels. | Avoid use of strong inhibitors of the bile salt export pump (BSEP) with RELYVRIO. If simultaneous use of a strong BSEP inhibitor like [[cyclosporine]] is deemed essential, exercise caution and monitor serum transaminases and bilirubin levels. | ||
*Aluminum-based Antacids: | |||
Avoid use of aluminum-based antacids with RELYVRIO and consider other acid lowering agents. | Avoid use of aluminum-based antacids with RELYVRIO and consider other acid lowering agents. | ||
*Avoid use of probenecid with RELYVRIO as it may affect renal excretion of sodium phenylbutyrate metabolites. | |||
*Phenylbutyrate is a pan-histone deacetylase (HDAC) inhibitor. Avoid use of other HDAC inhibitors with RELYVRIO. | |||
*In vitro studies showed that RELYVRIO is a substrate of OATP1B3. Avoid use of inhibitors of OATP1B3 with RELYVRIO. | |||
The possibility of RELYVRIO influencing the effects of other medications | The possibility of RELYVRIO influencing the effects of other medications: | ||
OAT1 Substrates | *OAT1 Substrates: | ||
If administered concurrently with RELYVRIO, the plasma levels of OAT1 substrate medications may rise. Avoid using OAT1 substrates that could result in severe toxicities or diminished efficacy with even a slight change in their plasma concentration when taking RELYVRIO. | If administered concurrently with RELYVRIO, the plasma levels of OAT1 substrate medications may rise. Avoid using OAT1 substrates that could result in severe toxicities or diminished efficacy with even a slight change in their plasma concentration when taking RELYVRIO. | ||
P-glycoprotein (P-gP) and Breast Cancer Resistance Protein (BCRP) Substrates | *P-glycoprotein (P-gP) and Breast Cancer Resistance Protein (BCRP) Substrates | ||
RELYVRIO has been shown to inhibit P-gP and BCRP in vitro. Plasma concentrations of P-gP and BCRP substrates may be increased if given concomitantly with RELYVRIO. Refrain from using P-glycoprotein (P-gP) and breast cancer resistance protein (BCRP) substrates concurrently with RELYVRIO, especially if a minor alteration in the substrate's plasma concentration could result in significant toxicities or a reduction in efficacy. | RELYVRIO has been shown to inhibit P-gP and BCRP in vitro. Plasma concentrations of P-gP and BCRP substrates may be increased if given concomitantly with RELYVRIO. Refrain from using P-glycoprotein (P-gP) and breast cancer resistance protein (BCRP) substrates concurrently with RELYVRIO, especially if a minor alteration in the substrate's plasma concentration could result in significant toxicities or a reduction in efficacy. | ||
Drugs that are substrates of CYP2C8, CYP1A2, CYP2B6, and CYP3A4isoenzymes | *Drugs that are substrates of CYP2C8, CYP1A2, CYP2B6, and CYP3A4isoenzymes | ||
RELYVRIO inhibits CYP2C8 and CYP2B6 isoenzymes in vitro. RELYVRIO induces CYP1A2, CYP2B6, and CYP3A4 in vitro. Plasma concentrations of substrates for these enzymes may be changed if given Simultaneously with RELYVRIO. Refrain from using medications that are processed by these CYP P450 isoenzymes, especially when even a slight alteration in the drug's plasma concentration could result in severe toxicities or diminished effectiveness. | RELYVRIO inhibits CYP2C8 and CYP2B6 isoenzymes in vitro. RELYVRIO induces CYP1A2, CYP2B6, and CYP3A4 in vitro. Plasma concentrations of substrates for these enzymes may be changed if given Simultaneously with RELYVRIO. Refrain from using medications that are processed by these CYP P450 isoenzymes, especially when even a slight alteration in the drug's plasma concentration could result in severe toxicities or diminished effectiveness. | ||
|useInPregnancyFDA=There are no available data on RELYVRIO use in pregnant women. | |useInPregnancyFDA=There are no available data on RELYVRIO use in pregnant women. | ||
| Line 85: | Line 85: | ||
|structure=RELYVRIO contains two active ingredients: sodium phenylbutyrate and taurursodiol. | |structure=RELYVRIO contains two active ingredients: sodium phenylbutyrate and taurursodiol. | ||
|PD=RELYVRIO does not cause large mean increases (>20 ms) in the QT interval. | |PD=RELYVRIO does not cause large mean increases (>20 ms) in the QT interval. | ||
|PK=Absorption: | |PK=*Absorption: | ||
Following oral administration of a single dose of RELYVRIO in healthy subjects under fasting conditions, sodium phenylbutyrate reaches a median Tmax of 0.5 hour. Taurursodiol reaches a median Tmax of 4.5 hours. | Following oral administration of a single dose of RELYVRIO in healthy subjects under fasting conditions, sodium phenylbutyrate reaches a median Tmax of 0.5 hour. Taurursodiol reaches a median Tmax of 4.5 hours. | ||
| Line 97: | Line 97: | ||
The majority of administered sodium phenylbutyrate (~80-100%) is excreted in the urine within 24 hours as the conjugated product, phenylacetylglutamine. | The majority of administered sodium phenylbutyrate (~80-100%) is excreted in the urine within 24 hours as the conjugated product, phenylacetylglutamine. | ||
|nonClinToxic=Carcinogenesis, Mutagenesis, Impairment of Fertility | |nonClinToxic=*Carcinogenesis, Mutagenesis, Impairment of Fertility | ||
Carcinogenesis: | Carcinogenesis: | ||
Studies to assess the carcinogenic potential of RELYVRIO have not been conducted. | Studies to assess the carcinogenic potential of RELYVRIO have not been conducted. | ||
Mutagenesis: | *Mutagenesis: | ||
The combination of sodium phenylbutyrate and taurursodiol (3:1 ratio of sodium phenylbutyrate and taurursodiol) was negative in in vitro (bacterial reverse mutation and mammalian cell chromosomal aberration) and in vivo (mouse micronucleus) assays. | The combination of sodium phenylbutyrate and taurursodiol (3:1 ratio of sodium phenylbutyrate and taurursodiol) was negative in in vitro (bacterial reverse mutation and mammalian cell chromosomal aberration) and in vivo (mouse micronucleus) assays. | ||
Impairment of Fertility: | *Impairment of Fertility: | ||
Oral administration of the combination of sodium phenylbutyrate and taurursodiol (0, 375, 750, or 1500 mg/kg/day) prior to and throughout mating in male and female rats and continuing to gestation day 7 in females resulted in no adverse effects on fertility. At the highest dose of the combination of sodium phenylbutyrate and taurursodiol tested, doses of sodium phenylbutyrate and taurursodiol were approximately 2 times the maximum recommended doses (6 g sodium phenylbutyrate and 2 g taurursodiol) in humans, based on body surface area (mg/m2). | Oral administration of the combination of sodium phenylbutyrate and taurursodiol (0, 375, 750, or 1500 mg/kg/day) prior to and throughout mating in male and female rats and continuing to gestation day 7 in females resulted in no adverse effects on fertility. At the highest dose of the combination of sodium phenylbutyrate and taurursodiol tested, doses of sodium phenylbutyrate and taurursodiol were approximately 2 times the maximum recommended doses (6 g sodium phenylbutyrate and 2 g taurursodiol) in humans, based on body surface area (mg/m2). | ||
Latest revision as of 23:34, 5 February 2024
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kosar Doraghi, M.D.[2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning: Gastrointestinal reactions
See full prescribing information for complete Boxed Warning.
Risk in Patients with Enterohepatic Circulation Disorders, Pancreatic Disorders, or Intestinal Disorders: In patients with disorders that interfere with bile acid circulation, consider consulting with a specialist. Monitor for new or worsening diarrhea in these patients. These conditions may also lead to decreased absorption of either of the components of RELYVRIO.
RELYVRIO has a high sodium content. In patients sensitive to salt intake, consider the amount of daily sodium intake in each dose of RELYVRIO and monitor appropriately.
|
Overview
RELYVRIO is a drug that containing the active ingredients sodium phenylbutyrate and taurursodiol that is FDA approved for the treatment of amyotrophic lateral sclerosis (ALS) in adults.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include diarrhea, abdominal discomfort, nausea, and upper respiratory tract infection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
The recommended dosage is 1 packet (3 g sodium phenylbutyrate and 1 g taurursodiol) administered orally or via feeding tube as follows:
- Initial dosage: 1 packet daily for the first 3 weeks
- maintenance dosage: 1 packet twice daily thereafter
- Empty contents of one packet in a cup containing 8 ounces of room temperature water and stir vigorously prior to administration.
- Take within 1 hour of preparation.
- Administer RELYVRIO before a snack or meal.
Off-Label Use and Dosage (Adult)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The safety and efficacy of RELYVRIO in pediatric patients have not been confirmed or established.
Off-Label Use and Dosage (Pediatric)
Contraindications
None.
Warnings
|
Warning: Gastrointestinal reactions
See full prescribing information for complete Boxed Warning.
Risk in Patients with Enterohepatic Circulation Disorders, Pancreatic Disorders, or Intestinal Disorders: In patients with disorders that interfere with bile acid circulation, consider consulting with a specialist. Monitor for new or worsening diarrhea in these patients. These conditions may also lead to decreased absorption of either of the components of RELYVRIO.
RELYVRIO has a high sodium content. In patients sensitive to salt intake, consider the amount of daily sodium intake in each dose of RELYVRIO and monitor appropriately.
|
Risk in Patients with Enterohepatic Circulation Disorders, Pancreatic Disorders, or Intestinal Disorders:
RELYVRIO includes taurursodiol, a bile acid. In individuals with conditions affecting bile acid circulation, there might be an elevated risk of worsening diarrhea, necessitating appropriate monitoring for this adverse reaction. Conditions such as pancreatic insufficiency, intestinal malabsorption, or disorders altering bile acid concentrations could result in reduced absorption of either RELYVRIO component. Given the varying severity of enterohepatic circulation, pancreatic, and intestinal disorders, it is advisable to seek consultation with a specialist. The study excluded patients with disorders affecting enterohepatic circulation (e.g., biliary infection, cholecystitis), severe pancreatic disorders (e.g., pancreatitis), and intestinal conditions impacting bile acid concentrations (e.g., ileal resection), hence lacking clinical experience in these situations.
Use in Patients Sensitive to High Sodium Intake:
RELYVRIO has a high salt content. Each initial daily dosage of one packet contains 464 mg of sodium; each maintenance dosage of two packets daily contains 928 mg of sodium. In patients sensitive to salt intake (e.g., those with heart failure, hypertension, or renal impairment), consider the amount of daily sodium intake in each dose of RELYVRIO and monitor appropriately.
Adverse Reactions
Clinical Trials Experience
In Study 1, involving 137 adult ALS patients randomized to either RELYVRIO or placebo for 24 weeks, safety was assessed. During this period, 6% of RELYVRIO-treated patients and 4% of placebo patients died, with the deaths attributed to ALS disease progression. The most common adverse reactions associated with RELYVRIO, occurring in at least 15% and at least 5% more than placebo, included diarrhea, abdominal pain, nausea, and upper respiratory tract infection. Gastrointestinal-related adverse reactions were more frequent in the first 3 weeks of treatment. Table 1 summarizes adverse reactions reported in over 5% of ALS patients taking RELYVRIO and at least 5% greater than those taking placebo in Study 1, with diarrhea and abdominal pain being the most prevalent.
Postmarketing Experience
There is limited information regarding RELYVRIO Postmarketing Experience in the drug label.
Drug Interactions
Potential for Other Drugs to Affect RELYVRIO:
- Bile Acid Sequestering Agents and Inhibitors of Bile Acid Transporters:
Bile acid sequestering agents (e.g., cholestyramine, colestipol, colesevelam) may interfere with the absorption of bile acids such as taurursodiol. Avoid use of bile acid sequestering agents with RELYVRIO and consider alternative cholesterol lowering agents.
Avoid use of strong inhibitors of the bile salt export pump (BSEP) with RELYVRIO. If simultaneous use of a strong BSEP inhibitor like cyclosporine is deemed essential, exercise caution and monitor serum transaminases and bilirubin levels.
- Aluminum-based Antacids:
Avoid use of aluminum-based antacids with RELYVRIO and consider other acid lowering agents.
- Avoid use of probenecid with RELYVRIO as it may affect renal excretion of sodium phenylbutyrate metabolites.
- Phenylbutyrate is a pan-histone deacetylase (HDAC) inhibitor. Avoid use of other HDAC inhibitors with RELYVRIO.
- In vitro studies showed that RELYVRIO is a substrate of OATP1B3. Avoid use of inhibitors of OATP1B3 with RELYVRIO.
The possibility of RELYVRIO influencing the effects of other medications:
- OAT1 Substrates:
If administered concurrently with RELYVRIO, the plasma levels of OAT1 substrate medications may rise. Avoid using OAT1 substrates that could result in severe toxicities or diminished efficacy with even a slight change in their plasma concentration when taking RELYVRIO.
- P-glycoprotein (P-gP) and Breast Cancer Resistance Protein (BCRP) Substrates
RELYVRIO has been shown to inhibit P-gP and BCRP in vitro. Plasma concentrations of P-gP and BCRP substrates may be increased if given concomitantly with RELYVRIO. Refrain from using P-glycoprotein (P-gP) and breast cancer resistance protein (BCRP) substrates concurrently with RELYVRIO, especially if a minor alteration in the substrate's plasma concentration could result in significant toxicities or a reduction in efficacy.
- Drugs that are substrates of CYP2C8, CYP1A2, CYP2B6, and CYP3A4isoenzymes
RELYVRIO inhibits CYP2C8 and CYP2B6 isoenzymes in vitro. RELYVRIO induces CYP1A2, CYP2B6, and CYP3A4 in vitro. Plasma concentrations of substrates for these enzymes may be changed if given Simultaneously with RELYVRIO. Refrain from using medications that are processed by these CYP P450 isoenzymes, especially when even a slight alteration in the drug's plasma concentration could result in severe toxicities or diminished effectiveness.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There are no available data on RELYVRIO use in pregnant women.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of RELYVRIO in women who are pregnant.
Labor and Delivery
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during RELYVRIO therapy
Nursing Mothers
There is no FDA guidance on the use of RELYVRIO in women who are nursing.
Pediatric Use
The safety and efficacy of RELYVRIO in pediatric patients have not been confirmed or established.
Geriatic Use
Among the 89 ALS patients administered RELYVRIO in Study 1, 28% were 65 years or older, with 4.5% being 75 years or older, and the oldest patient being 79 years old. There were no discernible variations in safety or efficacy between patients aged 65 and older and those under 65. While no specific differences in responses were noted between elderly and younger patients, the potential for increased sensitivity in some older individuals cannot be completely ruled out.
Gender
There is no FDA guidance on the use of RELYVRIO with respect to specific gender populations.
Race
There is no FDA guidance on the use of RELYVRIO with respect to specific racial populations.
Renal Impairment
No dose adjustment is needed for patients with mild renal impairment. Avoid use in patients with moderate or severe renal impairment.
Hepatic Impairment
No dose adjustment is needed for patients with mild hepatic impairment. Avoid use in patients with moderate or severe hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of RELYVRIO in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of RELYVRIO in patients who are immunocompromised.
Administration and Monitoring
Administration
RELYVRIO contains two active ingredients: sodium phenylbutyrate and taurursodiol.
For oral suspension: white to yellow powder provided in single-dose packets each containing 3 g sodium phenylbutyrate and 1 g taurursodiol.
The recommended initial dosage of RELYVRIO for oral suspension is 1 packet (3 g sodium phenylbutyrate and 1 g taurursodiol) daily for the first 3 weeks. After 3 weeks, increase to the maintenance dosage of 1 packet twice daily.
Monitoring
Empty contents of one packet in a cup containing 8 ounces of room temperature water and stir vigorously. Take orally or administer via feeding tube within 1 hour of preparation. The reconstituted suspension may be stored for up to 1 hour at room temperature. Discard any unused RELYVRIO reconstituted suspension after 1 hour.
Administer RELYVRIO before a snack or meal.
IV Compatibility
There is limited information regarding the compatibility of RELYVRIO and IV administrations.
Overdosage
There is limited information regarding RELYVRIO overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
The chemical designation for phenylbutyrate is 4-phenyl butyric acid sodium salt. Its molecular formula is C10H11NaO2, and its molecular weight is 186.2. The sodium phenylbutyrate chemical structure is:
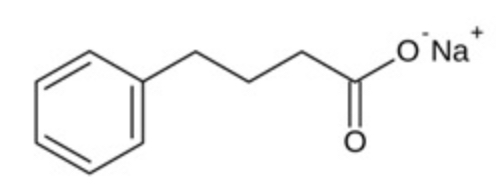
phenylbutyrate chemical structure Sodium phenylbutyrate is a white or yellow powder which decomposes at about 220°C. It is freely soluble in water and methanol; sparingly soluble in ethanol; and practically insoluble in methylene chloride, acetone, and diethyl ether.
Taurursodiol, also known as tauroursdeoxycholic acid, is an ambiphilic bile acid and is the taurine conjugate of ursodiol, also known as ursodeoxycholic acid. The chemical name of taurursodiol is 2-[(3α, 7β-dihydroxy-24-oxo-5β-cholan-24-yl) amino] ethane sulfonic acid, dihydrate. The molecular formula is C26H45NO6S . 2H2O and the molecular weight is 535.74 (dihydrate).
The taurursodiol chemical structure is:
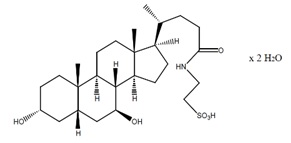
taurursodiol chemical structure
Taurursodiol is a white microcrystalline powder, practically odorless, with a bitter taste. It is freely soluble in ethyl alcohol, very slightly soluble in acetone and dioxane, sparingly soluble in water, and practically insoluble in ether and ethyl acetate.
RELYVRIO is a white to yellow powder for oral suspension that consists of fine to large granules. RELYVRIO is supplied in a packet containing 3 g sodium phenylbutyrate (equivalent to 2630 mg phenylbutyrate) and 1 g taurursodiol. Each packet contains 464 mg of sodium and also contains the following inactive ingredients: acacia, dextrates, dibasic sodium phosphate, maltodextrin, medium-chain triglycerides, mixed berry flavoring, other flavoring ingredients, silicon dioxide, sodium stearyl fumarate, sorbitol, and sucralose.
Mechanism of Action
Therapeutic effects in patients with ALS is unknown.
Structure
RELYVRIO contains two active ingredients: sodium phenylbutyrate and taurursodiol.
Pharmacodynamics
RELYVRIO does not cause large mean increases (>20 ms) in the QT interval.
Pharmacokinetics
- Absorption:
Following oral administration of a single dose of RELYVRIO in healthy subjects under fasting conditions, sodium phenylbutyrate reaches a median Tmax of 0.5 hour. Taurursodiol reaches a median Tmax of 4.5 hours.
Administration of RELYVRIO in the presence of a high-fat meal resulted in both significantly slower absorption and lower overall exposure of sodium phenylbutyrate.
Human plasma protein binding for sodium phenylbutyrate and taurursodiol is 82% and 98%, respectively.
No mass balance studies of sodium phenylbutyrate and taurursodiol have been conducted in humans to confirm the metabolic pathways and elimination routes. Phenylacetate was found to be a major metabolite of phenylbutyrate. Ursodiol and glyco-ursodiol were found as major metabolites of taurursodiol.
The majority of administered sodium phenylbutyrate (~80-100%) is excreted in the urine within 24 hours as the conjugated product, phenylacetylglutamine.
Nonclinical Toxicology
- Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis:
Studies to assess the carcinogenic potential of RELYVRIO have not been conducted.
- Mutagenesis:
The combination of sodium phenylbutyrate and taurursodiol (3:1 ratio of sodium phenylbutyrate and taurursodiol) was negative in in vitro (bacterial reverse mutation and mammalian cell chromosomal aberration) and in vivo (mouse micronucleus) assays.
- Impairment of Fertility:
Oral administration of the combination of sodium phenylbutyrate and taurursodiol (0, 375, 750, or 1500 mg/kg/day) prior to and throughout mating in male and female rats and continuing to gestation day 7 in females resulted in no adverse effects on fertility. At the highest dose of the combination of sodium phenylbutyrate and taurursodiol tested, doses of sodium phenylbutyrate and taurursodiol were approximately 2 times the maximum recommended doses (6 g sodium phenylbutyrate and 2 g taurursodiol) in humans, based on body surface area (mg/m2).
Clinical Studies
Study 1 involving 137 adult patients with amyotrophic lateral sclerosis (ALS), RELYVRIO demonstrated efficacy. Patients had a definite ALS diagnosis per revised El Escorial criteria, symptom onset within the past 18 months, and a slow vital capacity greater than 60% at screening. The study included 89 patients receiving RELYVRIO and 48 receiving placebo.
Baseline characteristics were generally similar between the groups. The primary efficacy endpoint, the rate of reduction in ALS Functional Rating Scale-Revised (ALSFRS-R) total scores from baseline to Week 24, showed a statistically significant difference in favor of RELYVRIO (p = 0.034). The ALSFRS-R scale assesses various functions related to ALS.
In a post hoc analysis, longer median overall survival was observed in patients initially randomized to RELYVRIO compared to those receiving placebo. However, caution is warranted in interpreting this exploratory analysis due to potential data limitations and confounding factors outside a controlled study setting.
How Supplied
RELYVRIO for oral suspension is supplied in single-dose packets of white to yellow powder containing 3 g sodium phenylbutyrate and 1 g taurursodiol as follows:
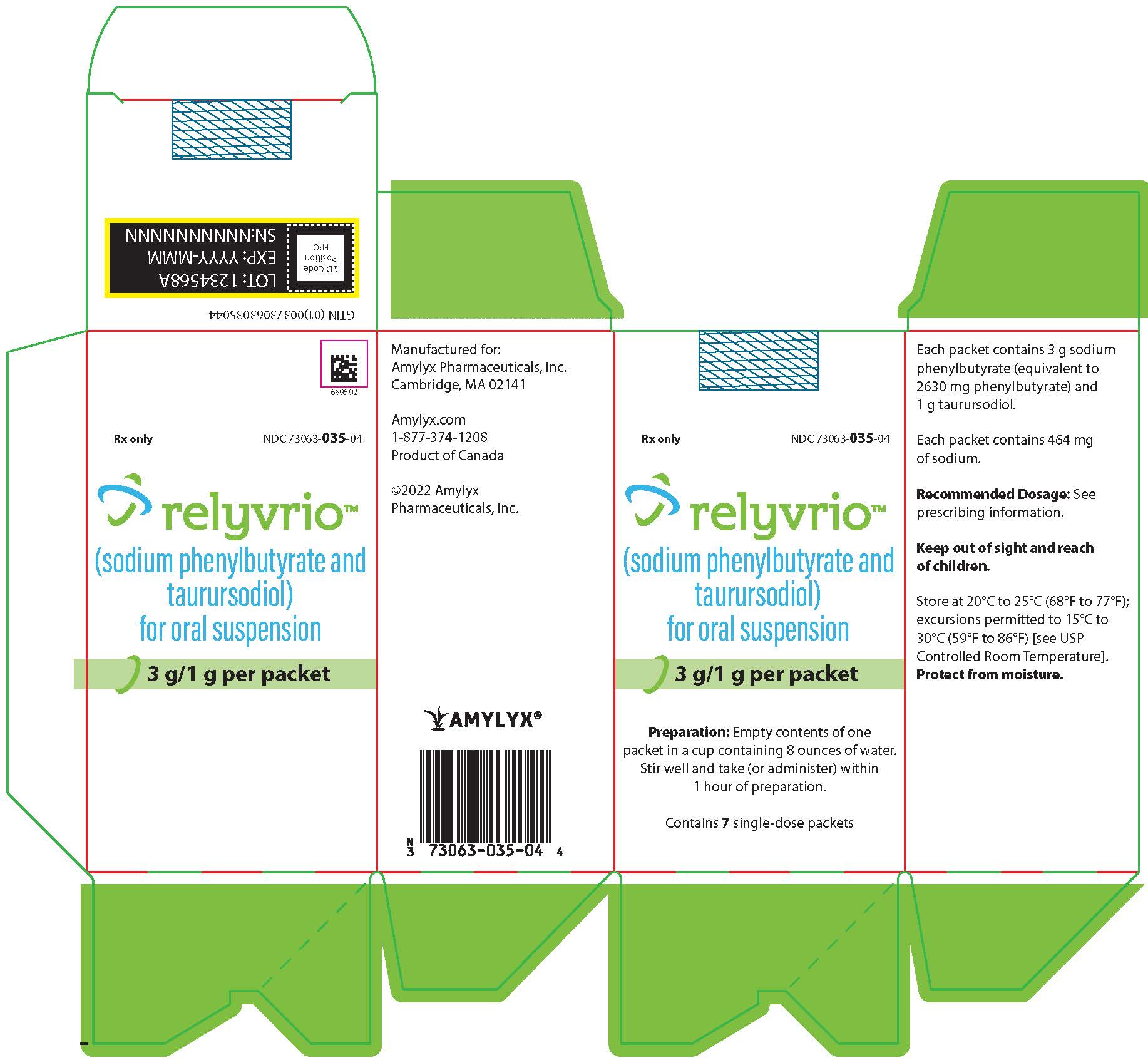
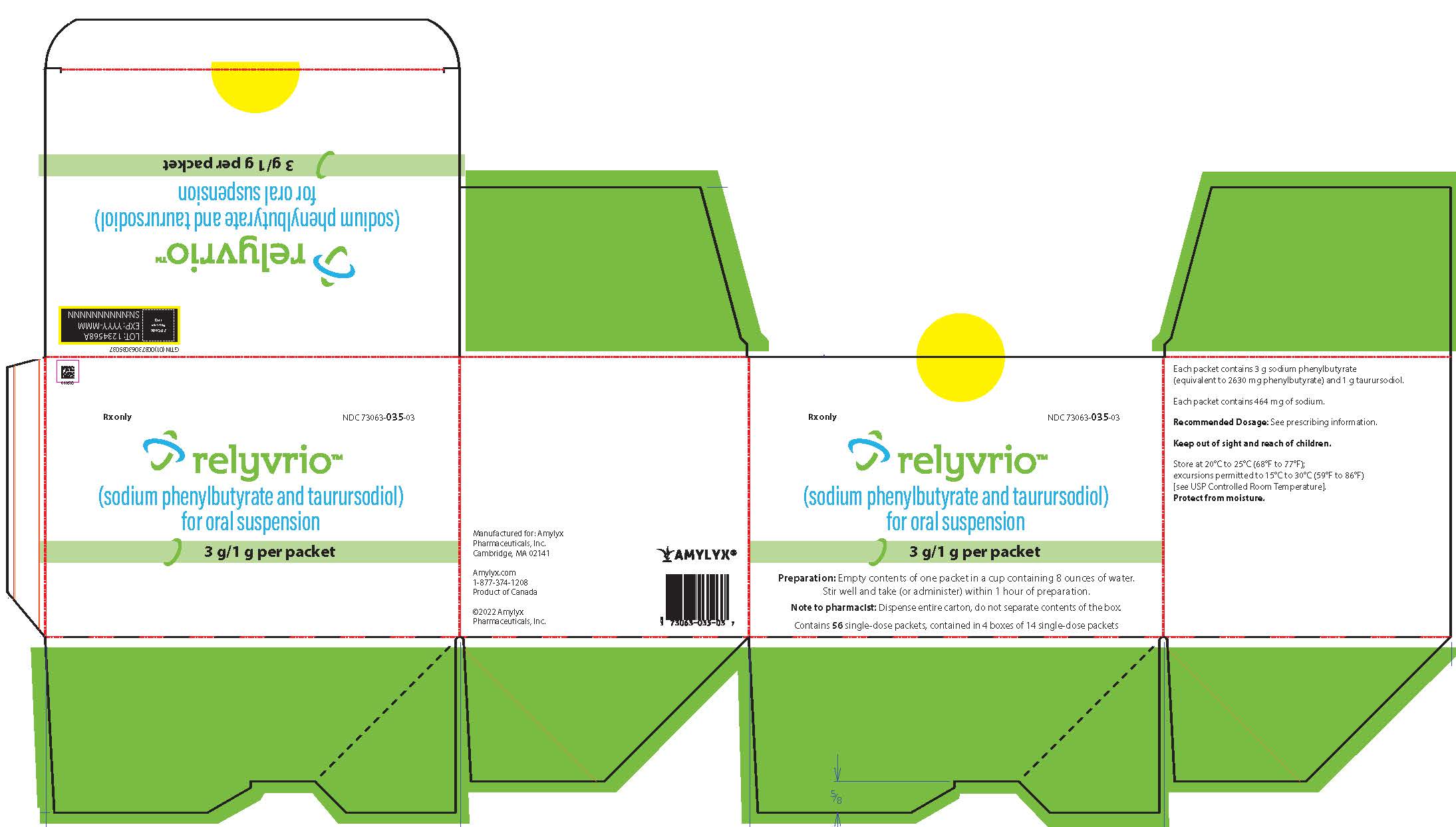
Carton of 7 single-dose packets (NDC 73063-035-04) Carton of 56 single-dose packets (Carton NDC 73063-035-03), contained in 4 boxes with 14 single-dose packets per box
Storage
Store at 20°C to 25°C (68°F to 77°F); Allow temperature variations within the range of 15°C to 30°C (59°F to 86°F). Avoid against moisture.
Images
Drug Images
{{#ask: Page Name::RELYVRIO |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
This image is provided by the National Library of Medicine. {{#ask: Label Page::RELYVRIO |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling.
Instruct patients or caregivers to empty the contents of one packet in a cup containing 8 ounces of room temperature water and stir vigorously. Advise them that RELYVRIO can be taken orally or administered via feeding tube, and to use or discard within 1 hour of preparation. Instruct to administer RELYVRIO before a snack or meal.
Advise patients with underlying medical conditions such as enterohepatic circulation, pancreatic, or intestinal disorders about the risks and benefits associated with the use of RELYVRIO. Instruct them to promptly inform their healthcare provider in case of new or worsening diarrhea.
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during RELYVRIO therapy
Precautions with Alcohol
Alcohol-RELYVRIO interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding RELYVRIO Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding RELYVRIO Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=RELYVRIO
|Pill Name=
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}