Wine fault
A wine fault or defect is an unpleasant characteristic of a wine often resulting from poor winemaking practices or storage conditions, and leading to wine spoilage. Many of the compounds that cause wine faults are already naturally present in wine but at insufficient concentrations to adversely affect it. In fact, depending on perception, these concentrations may impart positive characters to the wine. However when the concentration of these compounds greatly exceeds the sensory threshold, they replace or obscure the flavours and aromas that the wine should be expressing. Ultimately the quality of the wine is reduced, making it less appealing and sometimes undrinkable.
Oxidation
The oxidation of wine is perhaps the most common of wine faults, as the presence of oxygen and a catalyst are the only requirements for the process to occur. Oxidation can occur throughout the winemaking process, and even after the wine has been bottled. Anthocyanins, catechins, epicatechins and other phenols present in wine are those most easily oxidised [1], which leads to a loss of colour, flavour and aroma - sometimes referred to as flattening. In most cases compounds such as sulfur dioxide or erythorbic acid are added to wine by winemakers, which protect the wine from oxidation and also bind with some of the oxidation products to reduce their organoleptic effect[2]. Apart from phenolic oxidation, the ethanol present within wine can also be oxidised into other compounds responsible for flavour and aroma taints.
Acetaldehyde
Acetaldehyde is an intermediate product of yeast fermentation; however, it is more commonly associated with ethanol oxidation catalysed by the enzyme ethanol dehydrogenase. Acetaldehyde production is also associated with the presence of surface film forming yeasts and bacteria, such as acetic acid bacteria, which form the compound by the decarboxylation of pyruvate. The sensory threshold for acetaldehyde is 100-125 mg/L. Beyond this level it imparts a sherry type character to the wine which can also be described as green apple, sour and metallic. Acetaldehyde intoxication is also implicated in hangovers.
Acetic acid
Acetic acid in wine, often referred to as volatile acidity (VA) or vinegar taint, can be contributed by many wine spoilage yeasts and bacteria. This can be from either a by-product of fermentation, or due to the spoilage of finished wine. Acetic acid bacteria, such as those from the genera Acetobacter and Gluconobacter produce high levels of acetic acid. The sensory threshold for acetic acid is >700 mg/L, with concentrations greater than 1.2-1.3 g/L becoming unpleasant.
There are different opinions as to what level of volatile acidity is appropriate for higher quality wine. Although too high a concentration is sure to leave an undesirable, 'vinegar' tasting wine, some wine's acetic acid levels are developed to create a more 'complex', desirable taste.[3]
Ethyl acetate
Ethyl acetate is formed in wine by the esterification of ethanol and acetic acid. Therefore wines with high acetic acid levels are more likely to see ethyl acetate formation, but the compound does not contribute to the volatile acidity. It is a common microbial fault produced by wine spoilage yeasts, particularly Pichia anomala, Kloeckera apiculata, and Hanseniaspora uvarum. High levels of ethyl acetate are also produced by lactic acid bacteria and acetic acid bacteria. The sensory threshold for ethyl acetate is 150-200 mg/L. Levels below this can give an added richness and sweetness, whereas levels above impart nail polish remover, glue, or varnish type aromas.
Sulfur compounds
Sulfur is used as an additive throughout the winemaking process, primarily to stop oxidation as mentioned above but also as an antimicrobial agent. When managed properly in wine, its presence there is often undetected, however when used recklessly it can contribute to flavour and aroma taints which are very volatile and potent. Sulfur compounds typically have low sensory thresholds.
Sulfur dioxide
Sulfur dioxide is a common wine additive, used for its antioxidant and preservative properties. When its use is not managed well it can be overadded, with its perception in wine reminiscent of matchsticks, burnt rubber, or mothballs. Wines such as these are often termed sulfitic.
Hydrogen sulfide
Hydrogen sulfide (H2S) is generally thought to be a metabolic by-product of yeast fermentation in nitrogen limited environments. It is formed when yeast ferment via the sulfate reduction pathway. Fermenting wine is often supplemented with diammonium phosphate (DAP) as a nitrogen source to prevent formation. The sensory threshold for hydrogen sulfide is 40-50 μg/L, with levels above this imparting a distinct rotten egg aroma to the wine. Hydrogen sulfide can further react with wine compounds to form mercaptans and disulfides.
Mercaptans
Mercaptans (thiols) are produced in wine by the reaction of hydrogen sulfide with other wine components such as ethanol or sulfur containing amino acids, such as methionine. They can be formed if finished wine is allowed prolonged contact with the lees. This can be prevented by racking the wine. Mercaptans have a very low sensory threshold, around 1.5 µg/L[4], with levels above causing onion, rubber, and skunk type odours.
Dimethyl sulfide
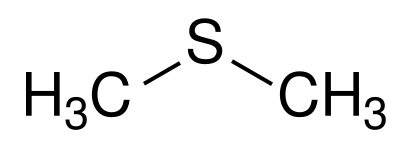
Dimethyl sulfide (DMS) is naturally present in most wines, probably from the breakdown of sulfur containing amino acids. It can also be formed when wines containing mercaptans are oxidised, which can occur during oak barrel ageing[5]. Like ethyl acetate, levels of DMS below the sensory threshold can have a positive effect on flavour, contributing to fruityness, fullness, and complexity. Levels above the sensory threshold of >30 µg/L in white wines and >50 µg/L for red wines, give the wine vegetative characteristics of cooked cabbage.
Environmental
Cork taint
Cork taint is a wine fault mostly attributed to the compound 2,4,6-trichloroanisole (TCA), although other compounds such as guaiacol, geosmin, 2-methylisoborneol, octen-3-ol, octen-3-one, 2,3,4,6-tetrachloroanisole, pentachloroanisole, and 2,4,6-tribromoanisole are also thought to be involved[6]. TCA most likely originates as a metabolite of mould growth on chlorine bleached wine corks and barrels. It causes earthy, mouldy, and musty aromas in wine that easily mask the natural fruit aromas, making the wine very unappealing. Wines in this state are often described as "corked". As cork taint has gained a wide reputation as a wine fault, other faults are often falsely identified as it.
Heat damage
Heat damaged wines are often casually referred to as cooked, which suggests how heat can affect a wine. The ideal storage temperature for wine is generally accepted to be 13°C (55°F). Wines that are stored at temperatures greatly higher than this will experience an increased ageing rate. Wines exposed to extreme temperatures will thermally expand, and may even push up between the cork and bottle and leak from the top. When opening a bottle of wine, if a track of wine is visible along the length of the cork, the cork is partially pushed out of the bottle, or wine is visible on the top of the cork while it is still in the bottle, it has most likely been heat damaged. Heat damaged wines often become oxidised, and red wines may take on a brick colour.
Reputedly, heat damage is the most widespread and common problem found in wines. It often goes unnoticed because of the prevalence of the problem, consumers don't know it's possible, and most often would just chalk the problem to poor quality or other factors.
Lightstrike
Lightstruck wines are those that have had excessive exposure to ultraviolet light, particularly in the range 325 to 450 nm[7]. Very delicate wines, such as Champagnes, are generally worst affected, with the fault causing a wet cardboard or wet wool type flavour and aroma. Red wines rarely becomes lightstruck because the phenolic compounds present within the wine protects it. Lightstrike is thought to be caused by sulfur compounds such as dimethyl sulfide. In France lightstrike is known as "goûts de lumière", which translates to tastes of light. The fault explains why wines are generally bottled in coloured glass, which blocks the ultraviolet light, and why wine should be stored in dark environments.
Ladybug taint
Some insects present in the grapes at harvest inevitably end up in the press and for the most part are inoffensive. Others, notably types of ladybugs, release unpleasant volatile compounds as a defensive mechanism when disturbed. In sufficient quantities this can affect the bouquet and taste of wines. With an olfactory detection threshold of a few ppb, the principal active compounds are methoxypyrazines, or just pyrazines, that are perceived as rancid peanut butter, bitter herbaceous, green bell pepper or cat pee. This is also a naturally occurring compound in Sauvignon grapes and so ladybugs taint has been known to make Rieslings taste like Sauvignon Blanc.
Microbiological
Brettanomyces (Dekkera)
The yeast Brettanomyces produces an array of metabolites when growing in wine, some of which are volatile phenolic compounds. Together these compounds are often referred to as "Brettanomyces character", or simply "Brett". The main constituents are listed below, with their sensory threshold and common sensory descriptors:
- 4-Ethylphenol (>140 µg/L): Band-aids, barnyard, horse stable, antiseptic
- 4-ethylguaiacol (>600 µg/L): Bacon, spice, cloves, smoky
- isovaleric acid: Sweaty saddle, cheese, rancidity
Geosmin
Geosmin is a compound with a very distinct earthy, musty, beetroot, even turnip flavour and aroma and has an extremely low sensory threshold of down to 10 parts per trillion. Its presence in wine is usually derived as metabolite from the growth of filamentous actinomycetes such as Streptomyces, and moulds such as Botritis cinerea and Penicillium expansum, on grapes. Wines affected by but not attributed to geosmins are often thought to have earthy properties due to terroir[8]. The geosmin fault occurs worldwide and has been found in recent vintages of red wines from Beaujolais, Bordeaux, Burgundy and the Loire in France. Geosmin is also thought to be a contributing factor in cork taint.
Lactic acid bacteria
Lactic acid bacteria have a useful role in winemaking converting malic acid to lactic acid in malolactic fermentation. However after this function has completed the bacteria may still be present within the wine, where they can metabolise other compounds and produce wine faults. Wines that have not undergone malolactic fermentation may be contaminated with lactic acid bacteria, leading to refermentation of the wine with it becoming turbid, swampy, and slightly effervescent or spritzy. This can be avoided by sterile filtering wine directly before bottling. Lactic acid bacteria can also be responsible for other wine faults such as those below.
Bitterness taint
Bitterness taint or amertume is rather uncommon and is produced by certain strains of bacteria from the genera Pediococcus, Lactobacillus, and Oenococcus. It begins by the degradation of glycerol, a compound naturally found in wine at levels of 5-8 g/L, via a dehydratase enzyme to 3-hydroxypropionaldehyde. During ageing this is further dehydrated to acrolein which reacts with the anthocyanins and other phenols present within the wine to form the taint[9]. As red wines contain high levels of anthocyanins they are generally more susceptible.
Diacetyl
Diacetyl in wine is produced by lactic acid bacteria, mainly Oenococcus oeni. In low levels it can impart positive nutty or caramel characters, however at levels above 5 mg/L it creates an intense buttery or butterscotch flavour, where it is perceived as a flaw. The sensory threshold for the compound can vary depending on the levels of certain wine components, such as sulfur dioxide. It can be produced as a metabolite of citric acid when all of the malic acid has been consumed. Diacetyl rarely taints wine to levels where it becomes undrinkable[10].
Geranium taint
Geranium taint, as the name suggests, is a flavour and aroma taint in wine reminiscent of geranium leaves. The compound responsible is 2-ethoxyhexa-3,5-diene, which has a low sensory threshold concentration of 0.1 mg/L[11]. In wine it is formed during the metabolism of potassium sorbate by lactic acid bacteria. Potassium sorbate is sometimes added to wine as a preservative against yeast, however its use is generally kept to a minimum due to the possibility of the taint developing. The production of the taint begins with the conversion of sorbic acid to the alcohol sorbinol. The alcohol is then isomerised in the presence of acid to 3,5-hexadiene-2-ol, which is then esterified with ethanol to form 2-ethoxy-3,5-hexadiene[11]. As ethanol is necessary for the conversion, the geranium taint is not usually found in must.
Mannitol
Mannitol is a polyol, and in wine it is produced by heterofermentative lactic acid bacteria, such as Lactobacillus brevis, by the reduction of fructose. Its perception is often complicated as it generally exists in wine alongside other faults, but it is usually described as viscous, ester-like combined with a sweet and irritating finish[9]. Mannitol is usually produced in wines that undergo malolactic fermentation with a high level of residual sugars still present.
Ropiness
Ropiness is manifested as an increase in viscosity and a slimey or fatty mouthfeel of a wine. In France the fault is known as "graisse", which translates to fat. The problem stems from the production of dextrins and polysaccharides by certain lactic acid bacteria, particularly of the genera Leuconostoc and Pediococcus.
Mousiness
tetrahydropyridine
Mousiness is a wine fault most often attributed to Brettanomyces but can also originate from the lactic acid bacteria Lactobacillus brevis, Lactobacillus fermentum, and Lactobacillus hilgardii[9]. The compounds responsible are lysine derivatives, mainly;
- 2-acetyl-3,4,5,6-tetrahydropyridine
- 2-acetyl-1,4,5,6-tetrahydropyridine
- 2-ethyltetrahydropyridine[12]
- 2-acetyl-1-pyrrolene
The taints are not volatile at the pH of wine, and therefore not obvious as an aroma. However, when mixed with the neutral pH of saliva they can become very apparent on the palate[13], especially at the back of the mouth, as mouse cage or mouse urine.
Refermentation
Refermentation, sometimes called secondary fermentation, is caused by yeasts refermenting the residual sugar present within bottled wine. It occurs when sweet wines are bottled in non-sterile conditions, allowing the presence of microorganisms. The most common yeast to referment wine is the standard wine fermentation yeast Saccharomyces cerevisiae, but has also been attributed to Schizosaccharomyces pombe and Zygosaccharomyces bailii[9]. The main issues associated with the fault include turbidity (from yeast biomass production), excess ethanol production (may violate labelling laws), slight carbonation, and some coarse odours. Refermentation can be prevented by bottling wines dry (with residual sugar levels <1.0g/L), sterile filtering wine prior to bottling, or adding preservative chemicals such as dimethyl dicarbonate. The Portuguese wine style known as "vinhos verdes" relies on this secondary fermentation in bottle to impart a slight spritziness to the wine.
See also
References
- ↑ duToit, W.J. (2005). Oxygen in winemaking: Part I. WineLand. URL accessed on 2 April 2006.
- ↑ Goode, Jamie (05/16/05). Oxidation. Wine International. URL accessed on 2 April 2006.
- ↑ Volatile Acidity - great article from Wine & Spirit mag
- ↑ Technical Bulletin - Sulfides in Wine. ETS Laboratories. URL accessed on 12 March 2006.
- ↑ Collings, Bill (01/28/02). Hydrogen sulfide and its derivatives. The British Columbia Amateur Winemakers Association. URL accessed on 12 March 2006.
- ↑ LaMar, Jim (09/25/02). Cork Taint. URL accessed on 12 March 2006.
- ↑ Drouhin, R.J. (01/23/98) Bottle Glass. URL Accessed 3 April 2006
- ↑ Kennel, Florence (14/12/05). Bordeaux boffin solves geosmin conundrum. Decanter.com. URL accessed on 2 April 2006.
- ↑ 9.0 9.1 9.2 9.3 duToit, M., Pretorius, I.S. (2000). “Microbial spoilage and preservation of wine: Using weapons from nature's own arsenal - A review”. South African Journal of Enology and Viticulture 21: 74-96.
- ↑ Gibson, George; Farkas, Mike Flaws and Faults in Wine. URL accessed on 12 March 2006.
- ↑ 11.0 11.1 Hühn, T; Sponholz, W.R. and Pulver, D. (1999). The influence of microorganisms in winemaking. (PDF) URL accessed on 2 April 2006.
- ↑ Marais, Johann Flavourful nitrogen containing wine constituents. Wynboer. URL accessed on 12 March 2006.
- ↑ Gawell, Richard Somellier, A Mouse Must Have Wee'd in My Wine!. aromadictionary.com. URL accessed on 12 March 2006.
External links
- Organoleptic defects in wine (PDF document)
- The influence of microorganisms in winemaking (PDF document)