Teniposide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Teniposide is an antineoplastic and a mitotic inhibitor that is FDA approved for the treatment of childhood acute lymphoblastic leukemia. Common adverse reactions include diarrhea, inflammatory disease of mucous membrane, nausea, vomiting, anemia, leukopenia, thrombocytopenia and infectious disease.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
NOTE: Contact of undiluted teniposide with plastic equipment or devices used to prepare solutions for infusion may result in softening or cracking and possible drug product leakage. This effect has not been reported with diluted solutions of teniposide.
- In order to prevent extraction of the plasticizer DEHP [di(2-ethylhexyl) phthalate], solutions of teniposide should be prepared in non-DEHP containing LVP containers such as glass or polyolefin plastic bags or containers.
- Teniposide solutions should be administered with non-DEHP containing intravenous administration sets.
- In one study, childhood ALL patients failing induction therapy with a cytarabine-containing regimen were treated with the combination of teniposide 165 mg/m2 and cytarabine 300 mg/m2 intravenously, twice weekly for 8 to 9 doses. In another study, patients with childhood ALL refractory to vincristine/prednisone-containing regimens were treated with the combination of teniposide 250 mg/m2 and vincristine 1.5 mg/m2 intravenously, weekly for 4 to 8 weeks and prednisone 40 mg/m2 orally for 28 days.
- Adequate data in patients with hepatic insufficiency and/or renal insufficiency are lacking, but dose adjustments may be necessary for patients with significant renal or hepatic impairment.
Preparation and Administration Precautions
- Caution should be exercised in handling and preparing the solution of teniposide. Several guidelines on proper handling and disposal of anticancer drugs have been published.1-4 Skin reactions associated with accidental exposure to teniposide may occur. To minimize the risk of dermal exposure, always wear impervious gloves when handling ampules containing teniposide. If teniposide solution contacts the skin, immediately wash the skin thoroughly with soap and water. If teniposide contacts mucous membranes, the membranes should be flushed immediately and thoroughly with water. More information is available in the references listed below.
Preparation for Intravenous Administration
- Teniposide must be diluted with either 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP, to give final teniposide concentrations of 0.1 mg/mL, 0.2 mg/mL, 0.4 mg/mL, or 1.0 mg/mL. Solutions prepared in 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP at teniposide concentrations of 0.1 mg/mL, 0.2 mg/mL, or 0.4 mg/mL are stable at room temperature for up to 24 hours after preparation. Teniposide solutions prepared at a final teniposide concentration of 1.0 mg/mL should be administered within 4 hours of preparation to reduce the potential for precipitation. Refrigeration of teniposide solutions is not recommended. Stability and use times are identical in glass and plastic parenteral solution containers.
- Although solutions are chemically stable under the conditions indicated, precipitation of teniposide may occur at the recommended concentrations, especially if the diluted solution is subjected to more agitation than is recommended to prepare the drug solution for parenteral administration. In addition, storage time prior to administration should be minimized and care should be taken to avoid contact of the diluted solution with other drugs or fluids. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Precipitation has been reported during 24-hour infusions of teniposide diluted to teniposide concentrations of 0.1 to 0.2 mg/mL, resulting in occlusion of central venous access catheters in several patients. Heparin solution can cause precipitation of teniposide, therefore, the administration apparatus should be flushed thoroughly with 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP before and after administration of teniposide.
Hypotension has been reported following rapid intravenous administration; it is recommended that the teniposide solution be administered over at least a 30- to 60-minute period. Teniposide should not be given by rapid intravenous injection.
- In a 24-hour study under simulated conditions of actual use of the product relative to dilution strength, diluent and administration rates, dilutions at 0.1 to 1.0 mg/mL were chemically stable for at least 24 hours. Data collected for the presence of the extractable DEHP [di(2-ethylhexyl) phthalate] from PVC containers show that levels increased with time and concentration of the solutions. The data appeared similar for 0.9% Sodium Chloride Injection, USP, and 5% Dextrose Injection, USP. Consequently, the use of PVC containers is not recommended.
- Similarly, the use of non-DEHP intravenous administration sets is recommended. Lipid administration sets or low DEHP-containing nitroglycerin sets will keep patient’s exposure to DEHP at low levels and are suitable for use. The diluted solutions are chemically and physically compatible with the recommended intravenous administration sets and LVP containers for up to 24 hours at ambient room temperature and lighting conditions. Because of the potential for precipitation, compatibility with other drugs, infusion materials, or intravenous pumps cannot be assured.
Stability
- Unopened ampules of teniposide are stable until the date indicated on the package when stored under refrigeration 2° to 8°C (36° to 46°F) in the original package. Freezing does not adversely affect the product.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Teniposide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Teniposide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Teniposide FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Teniposide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Teniposide in pediatric patients.
Contraindications
- Teniposide is generally contraindicated in patients who have demonstrated a previous hypersensitivity to teniposide and/or polyoxyl 35 castor oil.
Warnings
- Teniposide is a potent drug and should be used only by physicians experienced in the administration of cancer chemotherapeutic drugs. Blood counts, as well as renal and hepatic function tests, should be carefully monitored prior to and during therapy.
- Patients being treated with teniposide injection should be observed frequently for myelosuppression both during and after therapy. Dose-limiting bone marrow suppression is the most significant toxicity associated with teniposide therapy. Therefore, the following studies should be obtained at the start of therapy and prior to each subsequent dose of teniposide: hemoglobin, white blood cell count and differential, and platelet count. If necessary, repeat bone marrow examination should be performed prior to the decision to continue therapy in the setting of severe myelosuppression.
Physicians should be aware of the possible occurrence of a hypersensitivity reaction variably manifested by chills, fever, urticaria, tachycardia, bronchospasm, dyspnea, hypertension, orhypotension, rash, and facial flushing. This reaction may occur with the first dose of teniposide and may be life threatening if not treated promptly with antihistamines, corticosteroids, epinephrine, intravenous fluids, and other supportive measures as clinically indicated. The exact cause of these reactions is unknown. They may be due to the polyoxyl 35 castor oil componentof the vehicle or to teniposide itself. Patients who have experienced prior hypersensitivity reactions to teniposide are at risk for recurrence of symptoms and should only be retreatedreactions has been accomplished using measures described above. To date, there is no evidence to suggest cross-sensitization between teniposide and etoposide.
One episode of sudden death, attributed to probable arrhythmia and intractable hypotension, has been reported in an elderly patient receiving teniposide combination therapy for a nonleukemicmalignancy.
- Patients receiving teniposide treatment should be under continuous observation for at least the first 60 minutes following the start ofthe infusion and at frequent intervals thereafter. If symptoms or signs of anaphylaxis occur, the infusion should be stopped immediately, followed by the administration of epinephrine, corticosteroids, antihistamines, pressor agents, or volume expanders at the discretion of the physician. An aqueous solution of epinephrine 1:1000 and a source of oxygen should beavailable at the bedside.
- For parenteral administration, teniposide should be given only by slow intravenous infusion (lasting at least 30 to 60 minutes) since hypotension has been reported as a possible side effect of rapid intravenous injection, perhaps due to a direct effect of polyoxyl 35 castor oil. If clinically significant hypotension develops, the teniposide infusion should be discontinued. The blood pressure usually normalizes within hours in response to cessation of the infusion and administration of fluids or other supportive therapy as appropriate. If the infusion is restarted, a slower administration rate should be used and the patient should be carefully monitored.
- Acute central nervous system depression, hypotension, and metabolic acidosis have been observed in patients receiving investigational infusions of high-dose teniposide who were pretreated with antiemetic drugs. The depressant effects of the antiemetic agents and the alcohol content of the teniposide formulation may place patients receiving higher than recommended doses of teniposide a risk for central nervous system depression.
Adverse Reactions
Clinical Trials Experience
The table below presents the incidences of adverse reactions derived from an analysis of data contained within literature reports of 7 studies involving 303 pediatric patients in which teniposide was administered by injection as a single agent in a variety of doses and schedules for a variety of hematologic malignancies and solid tumors. The total number of patients evaluable for a given event was not 303 since the individual studies did not address the occurrence of each event listed. Five of these 7 studies assessed teniposide activity in hematologic malignancies, such as leukemia. Thus, many of these patients had abnormal hematologic status at start of therapy with teniposide and were expected to develop significant myelosuppression as an endpoint of treatment.

Hematologic Toxicity
- Teniposide, when used with other chemotherapeutic agents for the treatment of ALL, results in severe myelosuppression. Sepsis, sometimes fatal, may be a consequence of severe myelosuppression. Early onset of profound myelosuppression with delayed recovery can be expected when using the doses and schedules of teniposide necessary for treatment of refractory ALL, since bone marrow hypoplasia is a desired endpoint of therapy. The occurrence of acute non-lymphocytic leukemia (ANLL), with or without a preleukemic phase, has been reported in patients treated with teniposide in combination with other antineoplastic agents.
Gastrointestinal Toxicity
- Nausea and vomiting are the most common gastrointestinal toxicities, having occurred in 29% of evaluable pediatric patients. The severity of this nausea and vomiting is generally mild to moderate.
Hypotension
- Transient hypotension following rapid intravenous administration has been reported in 2% of evaluable pediatric patients. One episode of sudden death, attributed to probable arrhythmia and intractable hypotension, has been reported in an elderly patient receiving teniposide combination therapy for a non-leukemic malignancy.
- No other cardiac toxicity or electrocardiographic changes have been documented. No delayed hypotension has been noted.
Allergic Reactions
- Hypersensitivity reactions characterized by chills, fever, tachycardia, flushing, bronchospasm, dyspnea, rash, and blood pressure changes (hypertension or hypotension) have been reported to occur in approximately 5% of evaluable pediatric patients receiving intravenous teniposide. The incidence of hypersensitivity reactions to teniposide appears to be increased in patients with brain tumors and in patients with neuroblastoma.
Central Nervous System
- Neurotoxicity has been reported, including severe cases of neuropathy, in patients receiving vincristine sulfate and teniposide concomitantly.
- Acute central nervous system depression and hypotension have been observed in patients receiving investigational infusions of high-dose teniposide who were pretreated with antiemetic drugs. The depressant effects of the antiemetic agents and the alcohol content of the teniposide formulation may place patients receiving higher than recommended doses of teniposide at risk for central nervous system depression.
Alopecia
- Alopecia, sometimes progressing to total baldness, was observed in 9% of evaluable pediatric patients who received teniposide as single-agent therapy. It was usually reversible.
Other Adverse Reactions
- The following adverse reactions have been reported: headache, confusion, and asthenia. Headache and confusion were associated with hypersensitivity reactions.
Postmarketing Experience
There is limited information regarding Teniposide Postmarketing Experience in the drug label.
Drug Interactions
- In a study in which 34 different drugs were tested, therapeutically relevant concentrations of tolbutamide, sodium salicylate, and sulfamethizole displaced protein-bound teniposide in fresh human serum to a small but significant extent. Because of the extremely high binding of teniposide to plasma proteins, these small decreases in binding could cause substantial increases in free drug levels in plasma which could result in potentiation of drug toxicity. Therefore, caution should be used in administering teniposide to patients receiving these other agents. There was no change in the plasma kinetics of teniposide when coadministered with methotrexate. However, the plasma clearance of methotrexate was slightly increased. An increase in intracellular levels of methotrexate was observed in vitro in the presence of teniposide.
Use in Specific Populations
Pregnancy
- Teniposide may cause fetal harm when administered to a pregnant woman. Teniposide has been shown to be teratogenic and embryotoxic in laboratory animals. In pregnant rats, intravenous admin- istration of teniposide 0.1 to 3 mg/kg (0.6-18 mg/m2), every second day from day 6 to day 16 post coitum caused dose-related embryotoxicity and teratogenicity. Major anomalies included spinal and rib defects, deformed extremities, anophthalmia, and celosomia.
- There are no adequate and well-controlled studies in pregnant women. If teniposide is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant during therapy with teniposide.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Teniposide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Teniposide during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of teniposide therapy to the mother.
Pediatric Use
- Adverse events were evaluated in 7 studies involving 303 patients (age range 0.5 months to 20 years) who received teniposide as a single agent. No association between any particular age group and adverse effects was reported in any of these investigations.
Geriatic Use
There is no FDA guidance on the use of Teniposide in geriatric settings.
Gender
There is no FDA guidance on the use of Teniposide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Teniposide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Teniposide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Teniposide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Teniposide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Teniposide in patients who are immunocompromised.
Patients With Down Syndrome
- Patients with both Down syndrome and leukemia may be especially sensitive to myelosuppressive chemotherapy, therefore, initial dosing with teniposide should be reduced in these patients. It is suggested that the first course of teniposide should be given at half the usual dose. Subsequent courses may be administered at higher dosages depending on the degree of myelosuppression and mucositis encountered in earlier courses in an individual patient.
Administration and Monitoring
Administration
There is limited information regarding Teniposide Administration in the drug label.
Monitoring
There is limited information regarding Teniposide Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Teniposide and IV administrations.
Overdosage
- Acute central nervous system depression, hypotension, and metabolic acidosis have been observed in patients who were receiving higher than recommended doses of teniposide, and who were also pretreated with antiemetic drugs.
- There is no known antidote for teniposide overdosage. The anticipated complications of overdosage are secondary to bone marrow suppression. Treatment should consist of supportive care, including blood products and antibiotics as indicated.
Pharmacology
Mechanism of Action
- Teniposide is a phase-specific cytotoxic drug, acting in the late S phase or early G2 phase of the cell cycle, thus preventing cells from entering mitosis.
- Teniposide causes dose-dependent single- and double-stranded breaks in DNA and DNA-protein cross-links. The mechanism of action appears to be related to the inhibition of type II topoisomerase activity since teniposide does not intercalate into DNA or bind strongly to DNA. The cytotoxic effects of teniposide are related to the relative number of double-stranded DNA breaks produced in cells, which are a reflection of the stabilization of a topoisomerase II-DNA intermediate.
Structure
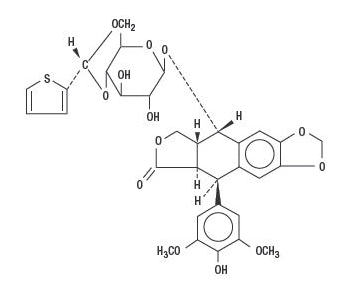
- Teniposide is a semisynthetic derivative of podophyllotoxin. The chemical name for teniposide is 4-demethylepipodophyllotoxin 9-4,6-0-(R)-2-thenylidene-β-D-glucopyranoside.
- Teniposide differs from etoposide, another podophyllotoxin derivative, by the substitution of a thenylidene group on the glucopyranoside ring.
- Teniposide has the following structural formula:
Pharmacodynamics
There is limited information regarding Teniposide Pharmacodynamics in the drug label.
Pharmacokinetics
Plasma drug levels declined biexponentially following intravenous infusion (155 mg/m2 over 1 to 2.5 hours) of teniposide given to 8 children (4-11 years old) with newly diagnosed acute lymphoblastic leukemia (ALL). The observed average pharmacokinetic parameters and associated coefficients of variation (CV%) based on a two-compartmental model analysis of the data are as follows:

- There appears to be some association between an increase in serum alkaline phosphatase or gamma glutamyl-transpeptidase and a decrease in plasma clearance of teniposide. Therefore, caution should be exercised if teniposide is to be administered to patients with hepatic dysfunction.
- In adults, at doses of 100 to 333 mg/m2/day, plasma levels increased linearly with dose. Drug accumulation in adult patients did not occur after daily administration of teniposide for 3 days. In pediatric patients, maximum plasma concentrations (Cmax) after infusions of 137 to 203 mg/m2 over a period of 1 to 2 hours exceeded 40 mcg/mL; by 20 to 24 hours after infusion plasma levels were generally <2 mcg/mL.
- Renal clearance of parent teniposide accounts for about 10% of total body clearance. In adults, after intravenous administration of 10 mg/kg or 67 mg/m2 of tritium-labeled teniposide, 44% of the radiolabel was recovered in urine (parent drug and metabolites) within 120 hours after dosing. From 4% to 12% of a dose is excreted in urine as parent drug. Fecal excretion of radioactivity within 72 hours after dosing accounted for 0% to 10% of the dose.
- Mean steady-state volumes of distribution range from 8 to 44 L/m2 for adults and 3 to 11 L/m2 for children. The blood-brain barrier appears to limit diffusion of teniposide into the brain, although in a study in patients with brain tumors, CSF levels of teniposide were higher than CSF levels reported in other studies of patients who did not have brain tumors.
- Teniposide is highly protein bound. In vitro plasma protein binding of teniposide is >99%. The high affinity of teniposide for plasma proteins may be an important factor in limiting distribution of drug within the body. Steady-state volume of distribution of the drug increases with a decrease in plasma albumin levels. Therefore, careful monitoring of children with hypoalbuminemia is indicated during therapy. Levels of teniposide in saliva, CSF, and malignant ascites fluid are low relative to simultaneously measured plasma levels.
- The pharmacokinetic characteristics of teniposide differ from those of etoposide, another podophyllotoxin. Teniposide is more extensively bound to plasma proteins and its cellular uptake is greater.
- Teniposide also has a lower systemic clearance, a longer elimination half-life, and is excreted in the urine as parent drug to a lesser extent than etoposide.
- In a study at St. Jude Children’s Research Hospital (SJCRH), 9 children with acute lymphocytic leukemia (ALL) failing induction therapy with a cytarabine-containing regimen, were treated with teniposide plus cytarabine. Three of these patients were induced into complete remission with durations of remission of 30 weeks, 59 weeks, and 13 years. In another study at SJCRH, 16 children with ALL rrefractory to vincristine/prednisone-containing regimens were treated with teniposide plus vincristine and prednisone. Three of these patients were induced into complete remission with durations of remission of 5.5, 37, and 73 weeks. In these 2 studies, patients served as their own control based on the premise that long-term complete remissions could not be achieved by re-treatment with drugs to which they had previously failed to respond.
Nonclinical Toxicology
There is limited information regarding Teniposide Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Teniposide Clinical Studies in the drug label.
How Supplied

Storage
- Store the unopened ampules under refrigeration 2° to 8°C (36° to 46°F). Retain in original package to protect from light.
Images
Drug Images
{{#ask: Page Name::Teniposide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Teniposide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Teniposide Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Teniposide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Teniposide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.