Ventricular septal defect pathophysiology
| https://https://www.youtube.com/watch?v=e7ObXrdtXek%7C350}} |
|
Ventricular septal defect Microchapters | |
|
Differentiating Ventricular Septal Defect from other Diseases | |
|---|---|
|
Diagnosis | |
|
ACC/AHA Guidelines for Surgical and Catheter Intervention Follow-Up | |
|
Case Studies | |
|
Ventricular septal defect pathophysiology On the Web | |
|
American Roentgen Ray Society Images of Ventricular septal defect pathophysiology | |
|
Risk calculators and risk factors for Ventricular septal defect pathophysiology | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] and Leida Perez, M.D.; Associate Editor(s)-in-Chief: Keri Shafer, M.D. [2]; Priyamvada Singh, M.B.B.S; Omar Toubat
Overview
In ventricular septal defect, a persistent opening in the upper interventricular septum resulting from failure of fusion with the aortic septum allows blood to flow from the high pressure left ventricle into the low pressure chamber or right ventricle. Disruption of the septation process, from inherited perturbations during embryological development or acquired cardiac injury, may result in ventricular septal defects (VSDs).
Pathophysiology
Embryology of VSD
The goal of ventricular septation is to permanently divide a single ventricular cavity into unique right and left chambers. Successful division of the ventricles necessitates a continuous barrier to ensure pulmonary and systemic flow separation in the developed heart. The true interventricular septum is a heterogenous structure composed of a muscular segment and a membranous segment. Disruption of the septation process, from inherited perturbations during embryological development or acquired cardiac injury, may result in ventricular septal defects (VSDs). The most commonly surgically corrected VSDs arise in the fibrous membranous ventricular septum.[1]
Septation of the primitive ventricle into distinct right and left ventricular chambers begins shortly after cardiac looping. In the early stages of bilateral ventricular formation there is a large interventricular communication known as the primary interventricular foramen.[2] However, this defect is temporary and begins to narrow as ventricular ballooning creates an upward muscular growth from the floor of the ventricle.[3] The growth of the primitive muscular septum from its apical origin towards the endocardial cushion arrests before closure of the defect is complete, leaving a secondary interventricular foramen.[4] Eventually the secondary interventricular foramen in sealed, accomplishing the bilateral division of the right and left ventricular chambers. Closure of the secondary interventricular foramen requires the proper convergence of three different tissues:[5]
- The muscular interventricular septum
- The endocardial cushion
- The bulbar septum
Together, the endocardial cushion and the bulbar septum contribute to the formation of the membranous septum, which will intersect the muscular septum and complete the process of ventricular septation.[5]
VSDs develop in the membranous or muscular portions of the ventricular septum. Congenital muscular septal defects can emerge because of non-compaction of the muscular septum, leaving one of many interventricular communications in the postnatal heart.[4] Likewise, improper positioning or growth of any component of the membranous septum also results in abnormal septation.[4] Appropriate ventricular septation is a coordinated effort involving the spatiotemporal placement of several different tissue components. Morphological defects in this complex process are varied and can occur at any point during septation, accounting for the phenotypic dynamism in VSDs.
Anatomy of Ventricular Septum
Click here to learn more about the anatomy of the ventricular septum.
Diagram of VSD

Please click here to learn more about the normal ventricular septum anatomy.
Factors Affecting the Pathophysiology of VSD
- The subsequent natural history and pathophysiology depends on
- The size of the defect
- The magnitude of left-to-right shunting.
- Small defects (QP/QS less than 1.5) maybe asymptomatic, but with the high risk for bacterial endocarditis.
- Large defects are associated with left ventricular failure.
- Chronic but more moderate left-to-right shunts may lead to pulmonary vascular disease and right sided failure.
The primary variable is the size of the defect. As a child grows, the relative size of the defect may decrease and the defect may even close spontaneously in early childhood.
During the first few months of life the PVR decreases, and the magnitude of left-to-right shunt increases. After the first few months the degree of shunting is dependent on the size of the defect.
Presentations in the Adult or Adolescent
a) Small defect without significant left-to-right shunting
b) Large defect with severe pulmonary hypertension and cyanosis due to right-to-left shunt.
c) Large defect with a large left-to-right shunt that has induced secondary infundibular stenosis (tough to differentiate from tetralogy of Fallot).
Small VSDs
A high resistance to flow across the VSD due to the large pressure difference between the two ventricles. There is a small left-to-right shunt (Qp/Qs < 1.5) and a normal ratio of PA to systemic pressures.
There is little or no increase in the pulmonary vascular resistance. A holosystolic murmur is present due to the pressure gradient across the defect. The majority of these defects close during the first three years of life.
Medium-Sized VSDs
There is a moderate left-to-right shunt present (Qp/Qs = 1.5-2.0) that still has some resistance to flow across the defect. There is also volume overload of the LA and the LV and LVH. There may therefore be a mid diastolic mitral murmur and a third heart sound (S3). The ratio of the PA systolic pressure to the systemic pressure is < 5.
The area of the defect is usually less than 1 cm2/m2 of body surface area and is unusual for this group to have a marked increase in PVR. In some cases and depending on the type of VSD, as the child becomes older, the relative size of the defect will decrease.
Large VSDs
There is a large defect on the ventricular septum, > 1 cm2/m2 of BSA, with a large shunt left-to-right (Qp/Qs is > 2), causing volume overload of the LV, which may result in its failure. The defect may approximate the size of the aortic orifice.
The ratio of the PA pressure to the systemic pressure is > 5. Produce the same clinical findings as moderate sized VSD but also pulmonary hypertension.
There is rarely spontaneously closure of the defect, and these patients either die, or progress to adolescence or adulthood with severe pulmonary hypertension or with secondary protective infundibular pulmonary stenosis.
In the group with severe pulmonary hypertension, the left-to-right shunt decreases and the degree of right-to-left shunting increases with accompanying cyanosis (i.e. they develop Eisenmenger's syndrome).
Protective infundibular stenosis may also result in reversal of the shunt, and may be indistinguishable clinically from tetralogy of Fallot.
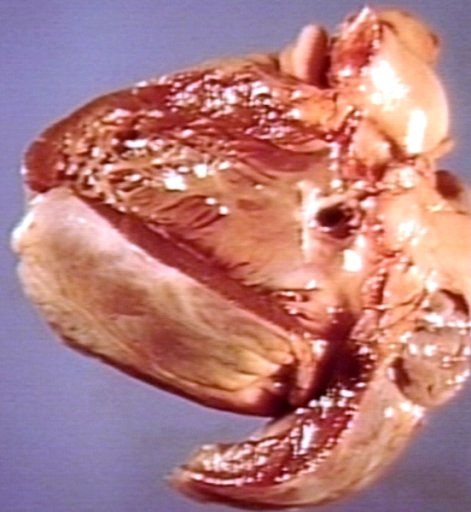
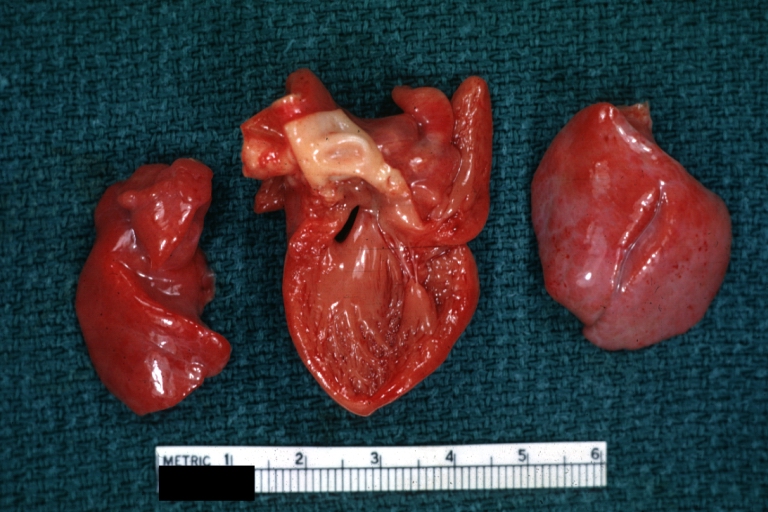
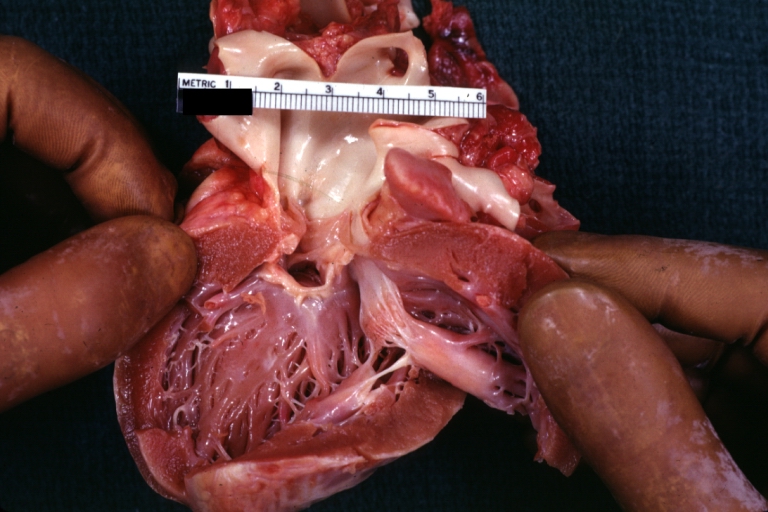
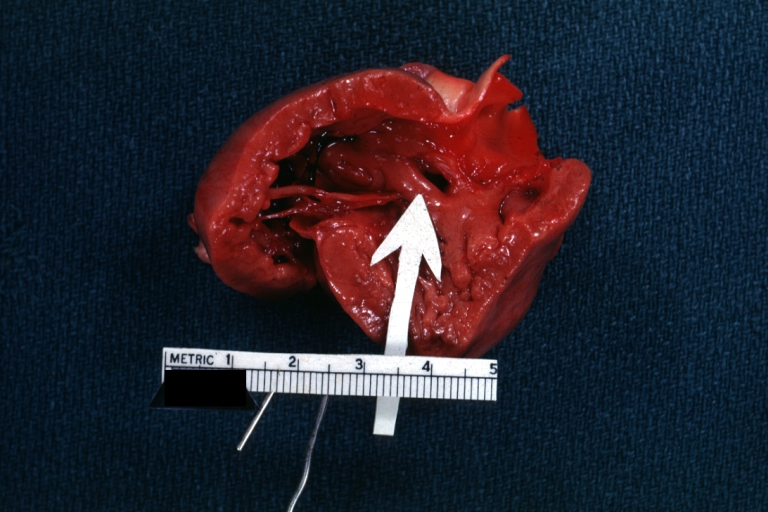
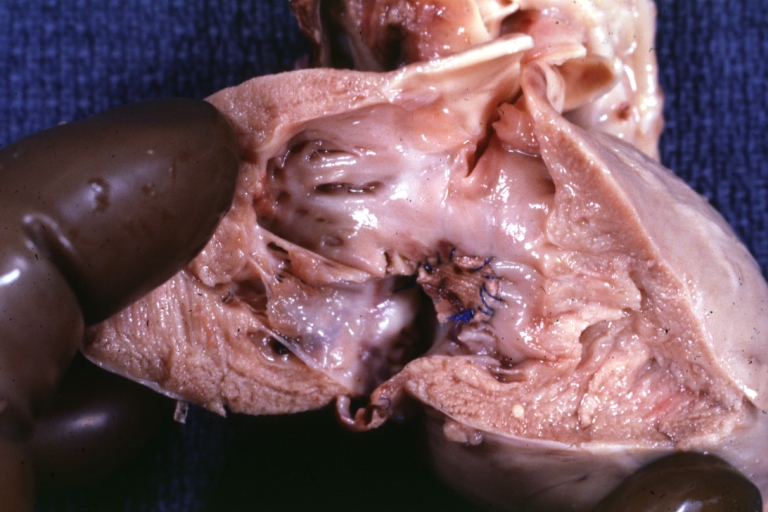
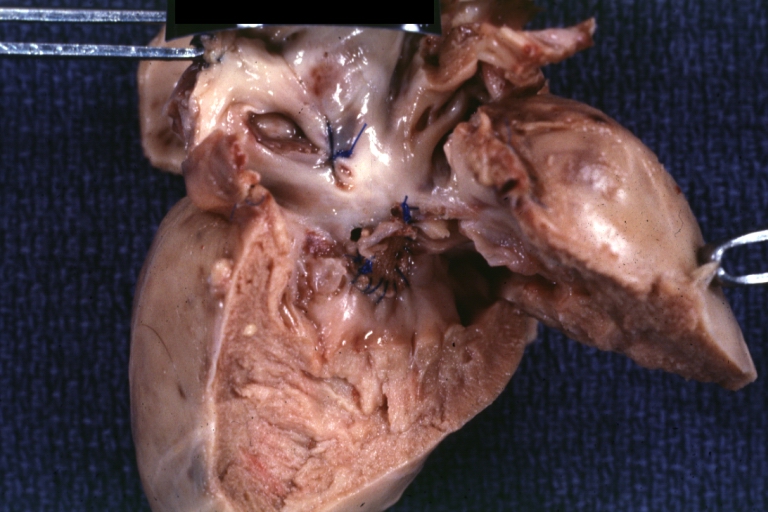
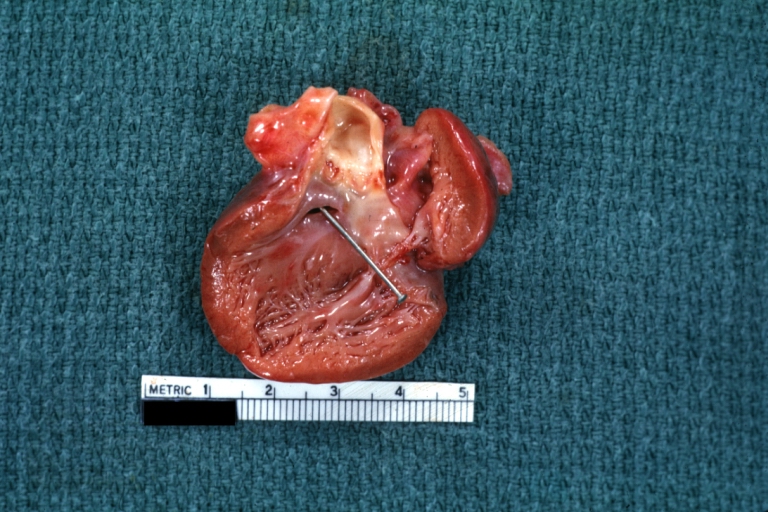
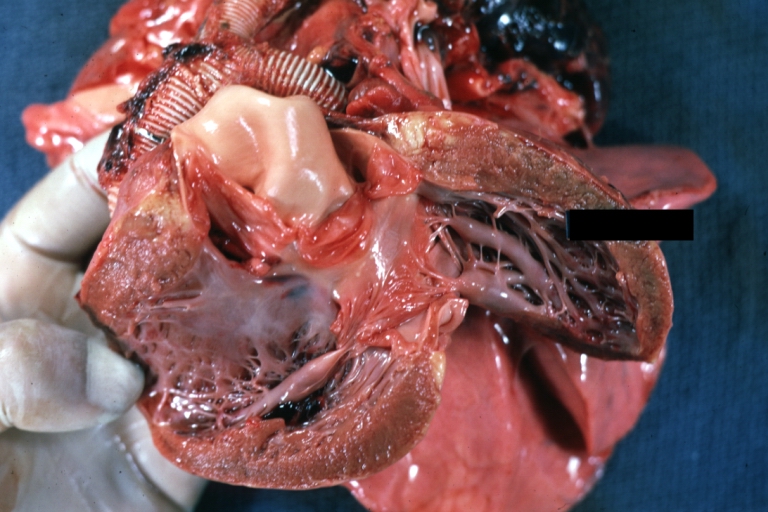
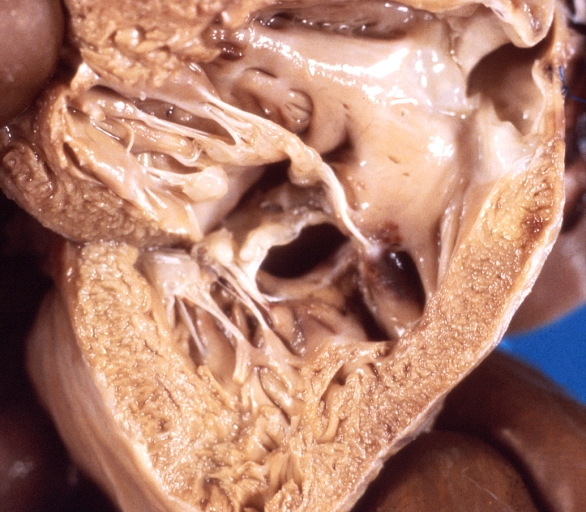
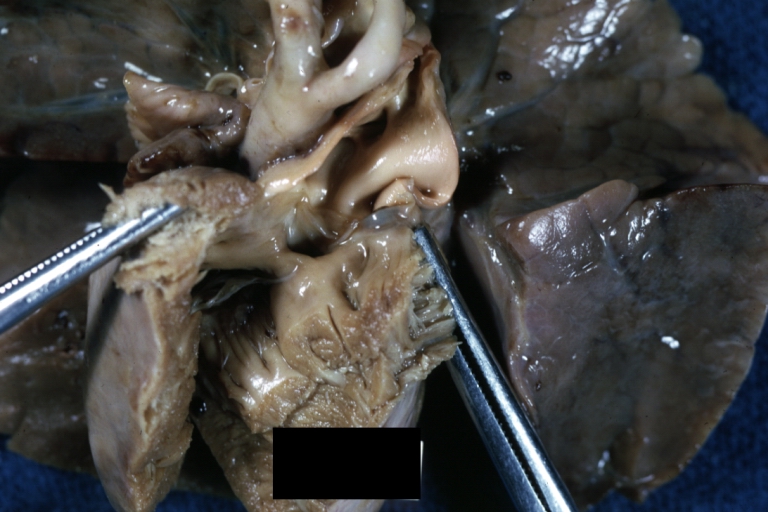
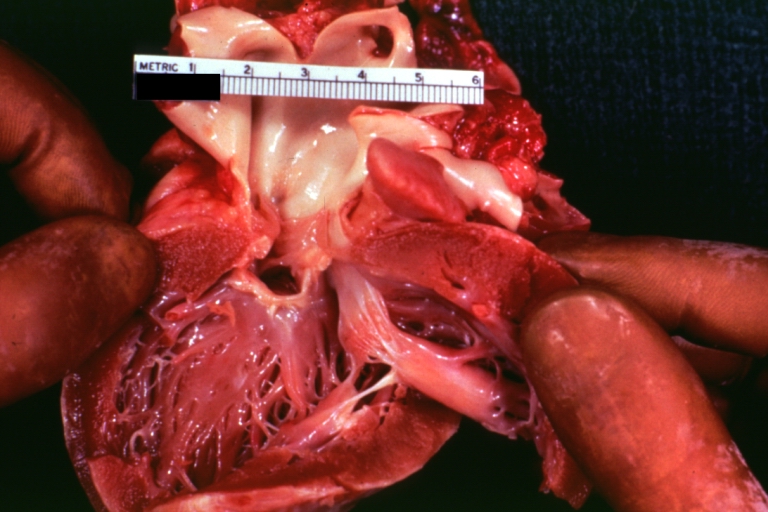
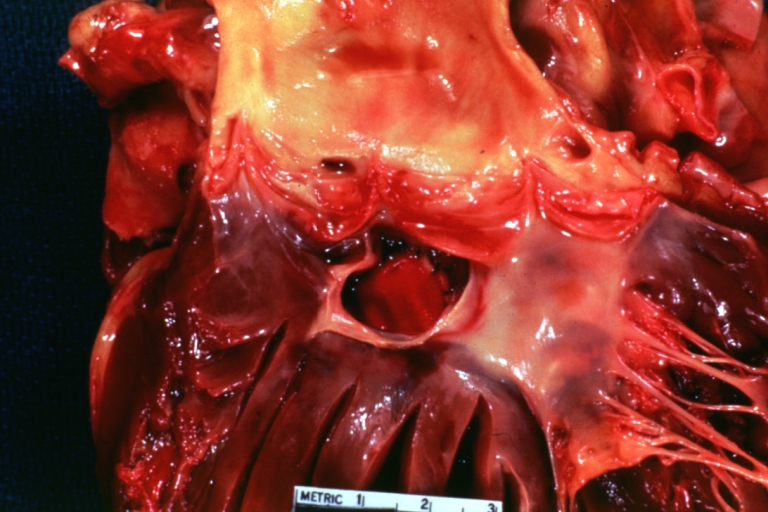
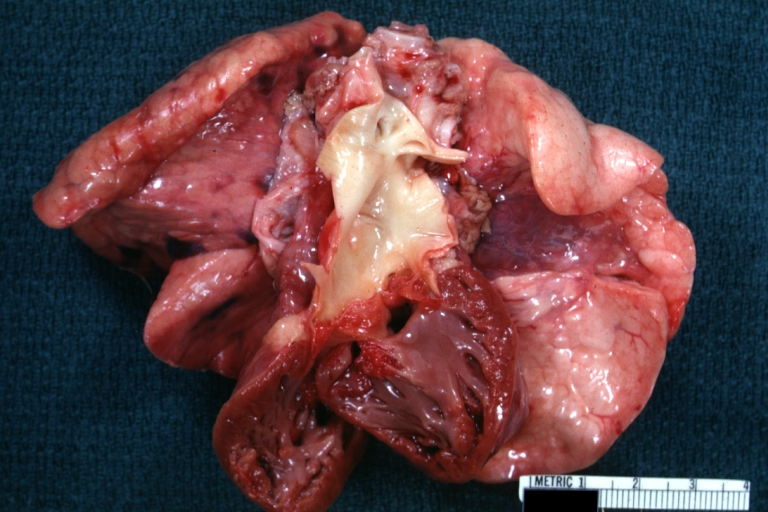
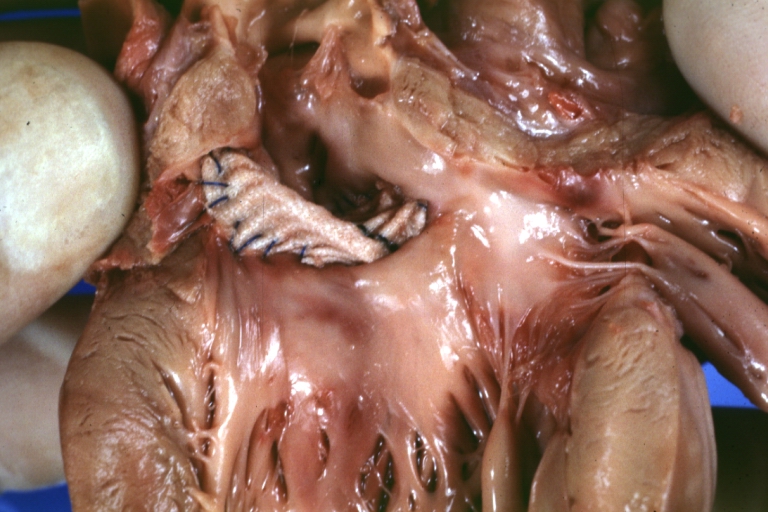
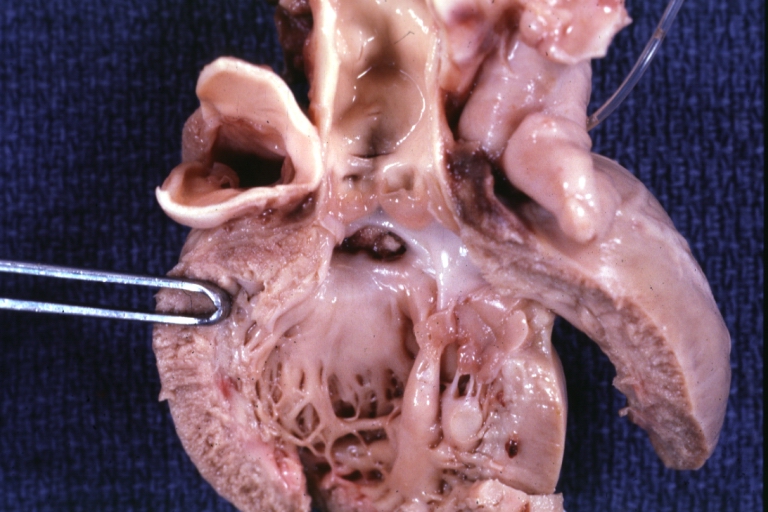
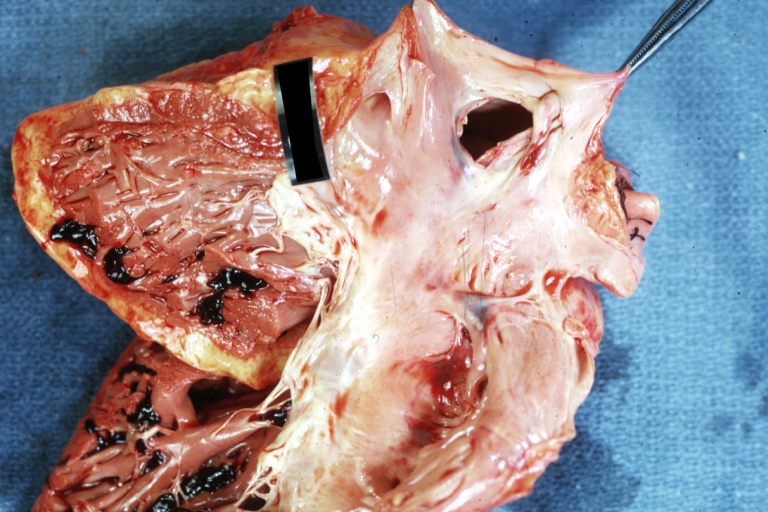
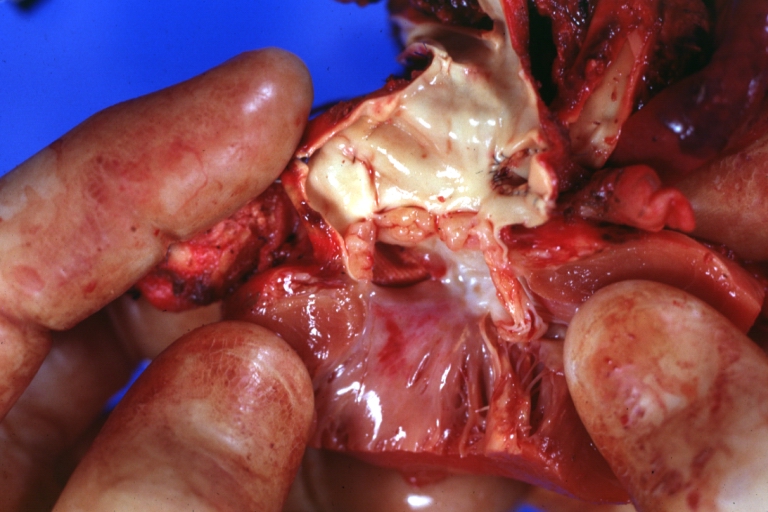
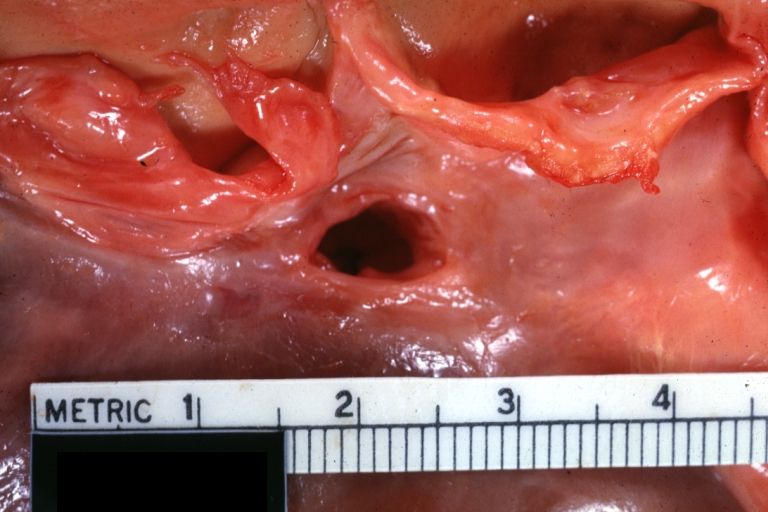
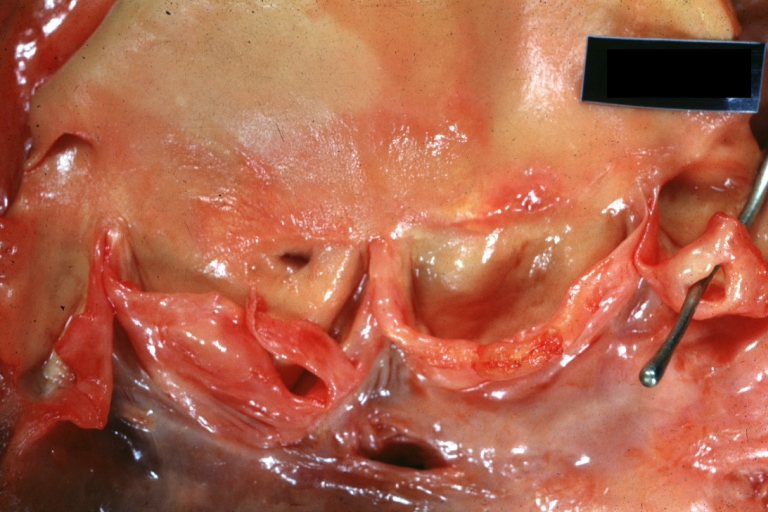
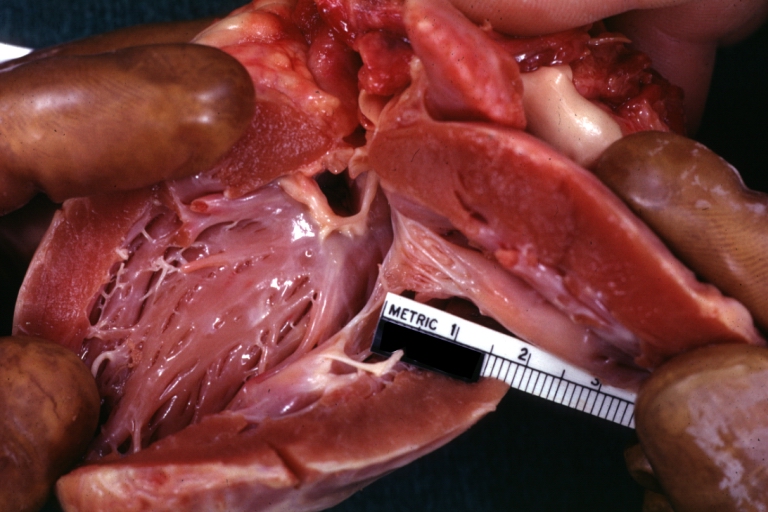
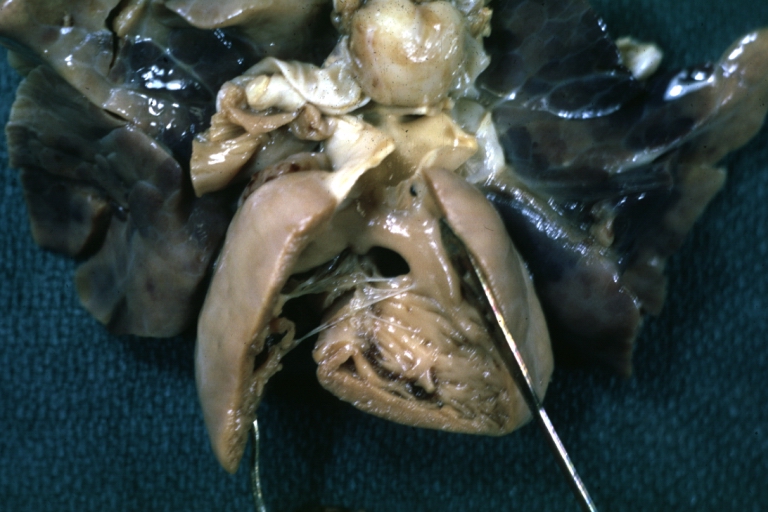
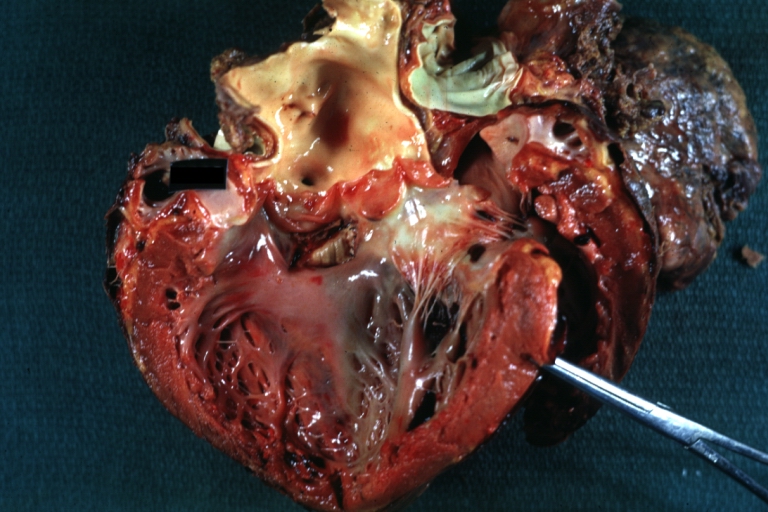
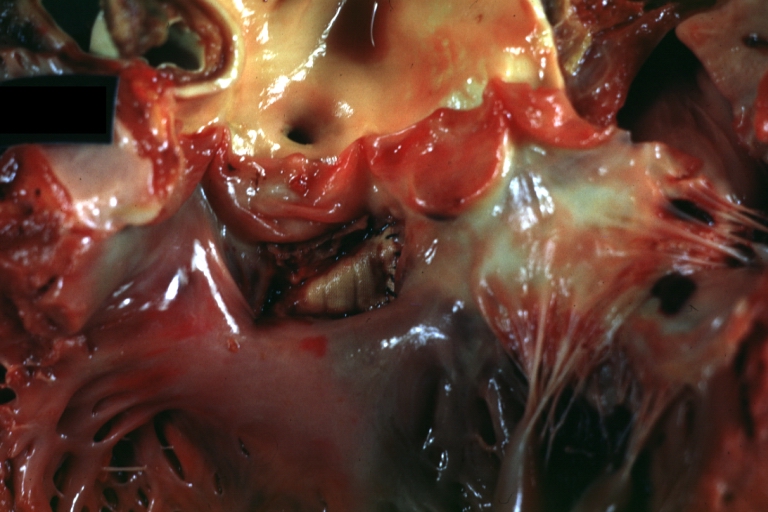
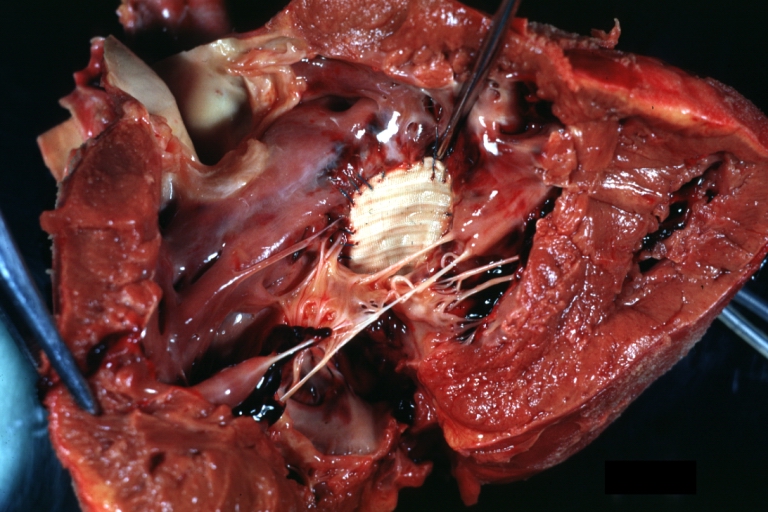
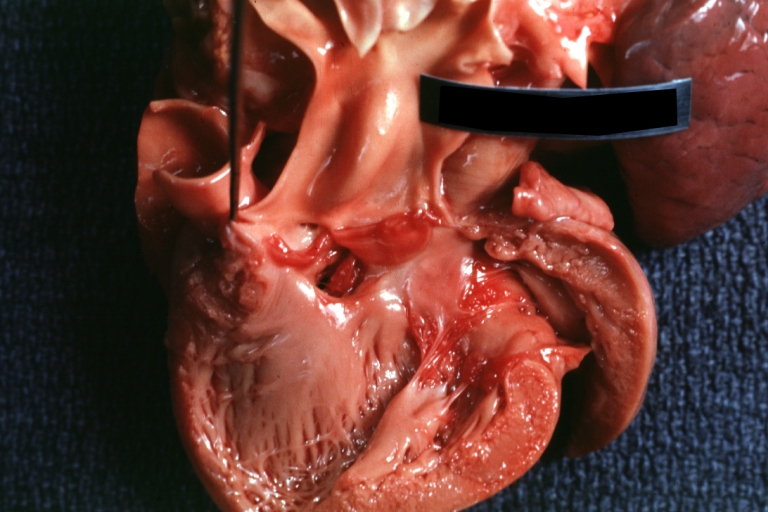
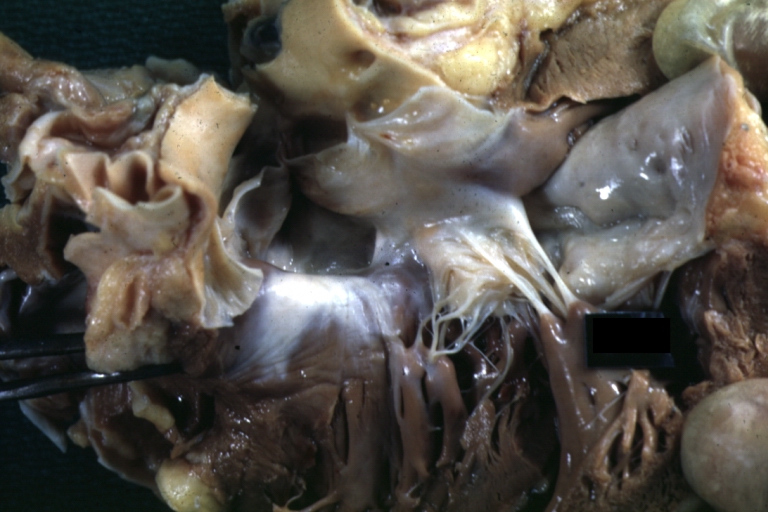
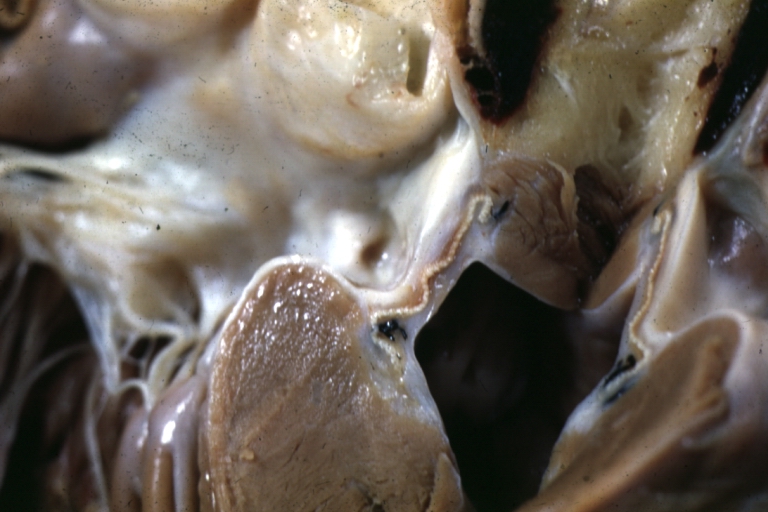
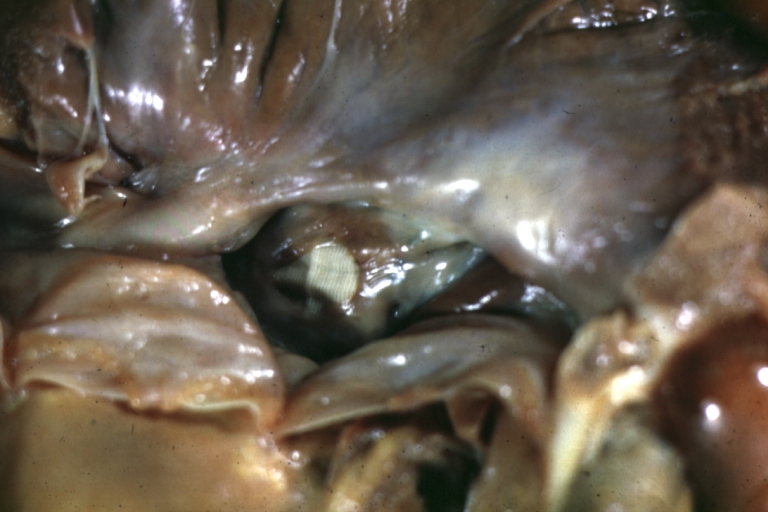
Gross Pathology
-
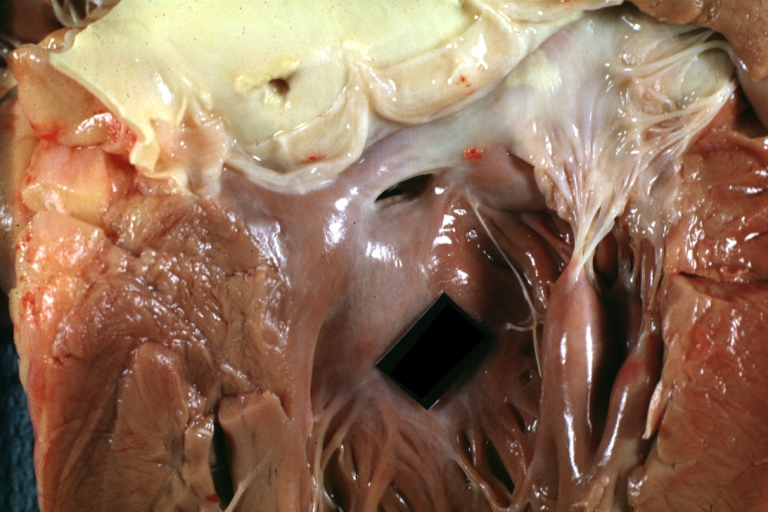
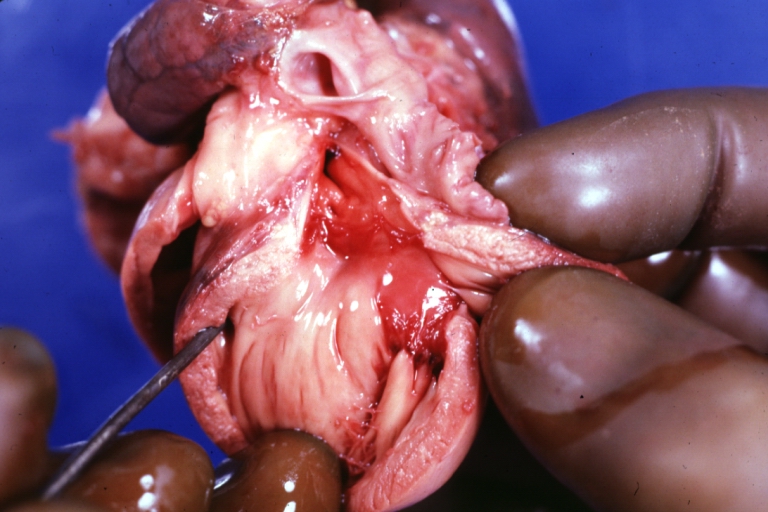
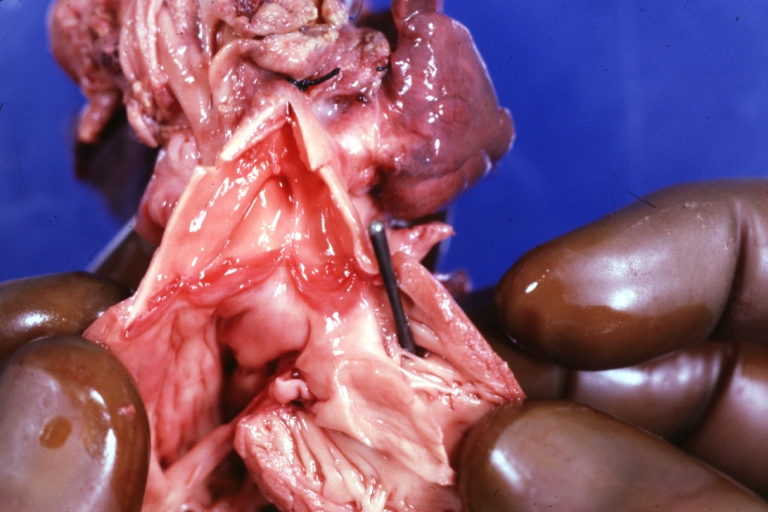
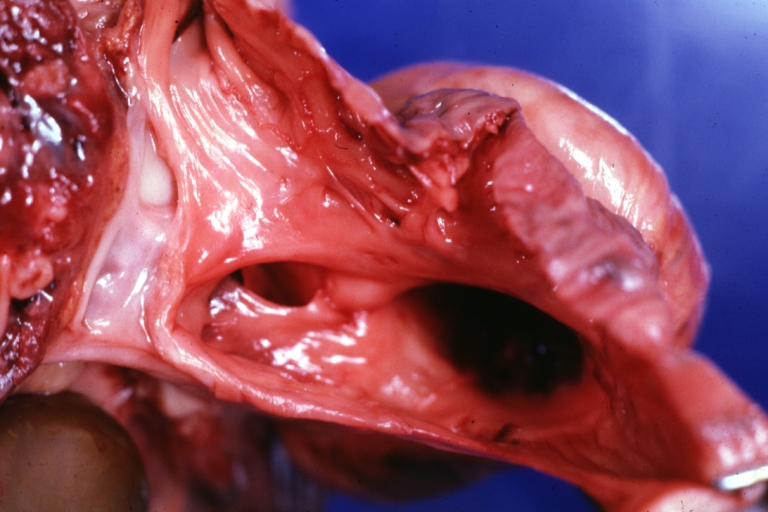
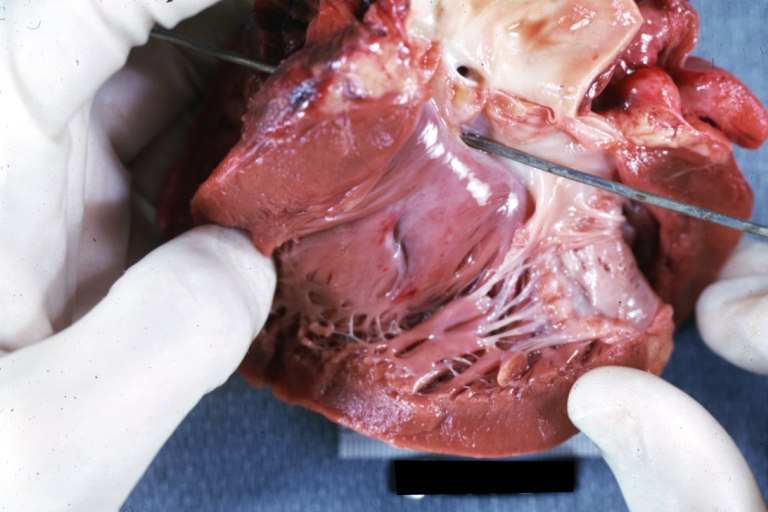
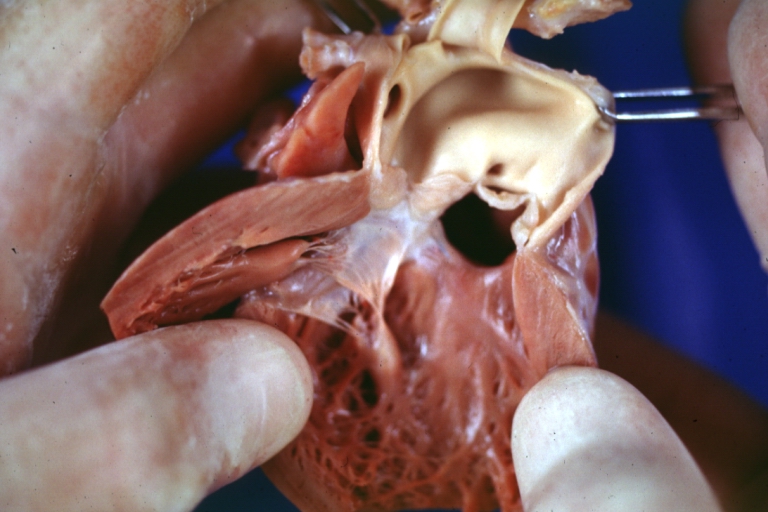
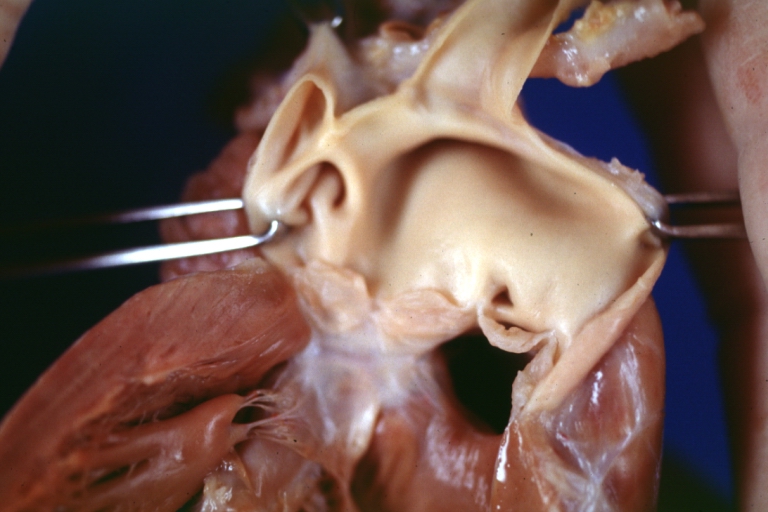
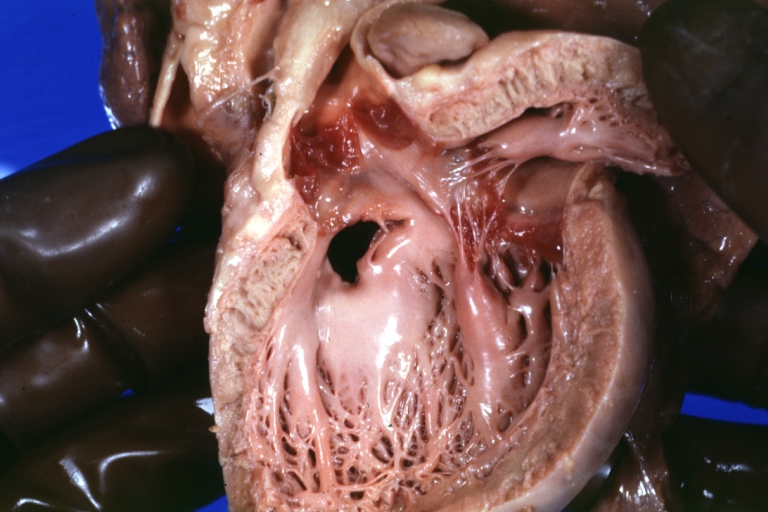
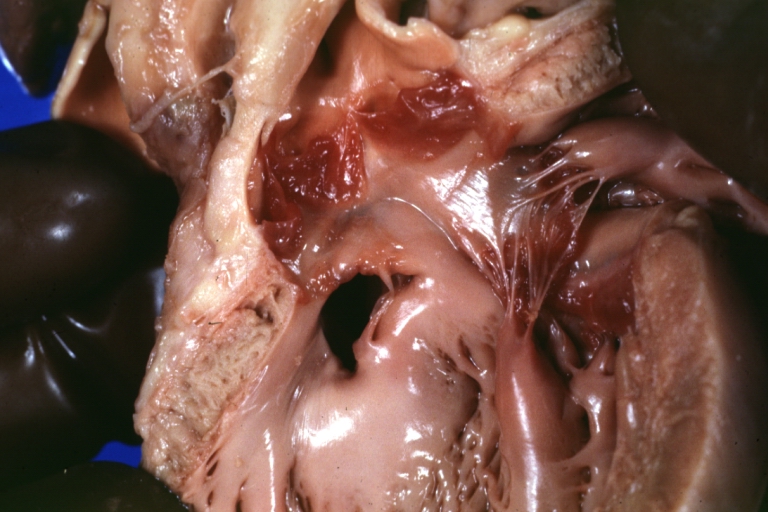
Ventricular septal defect, view from left ventricle
-
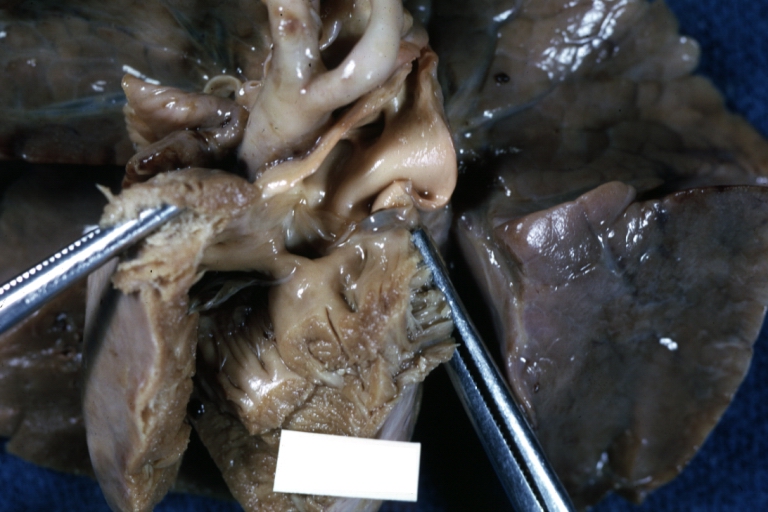
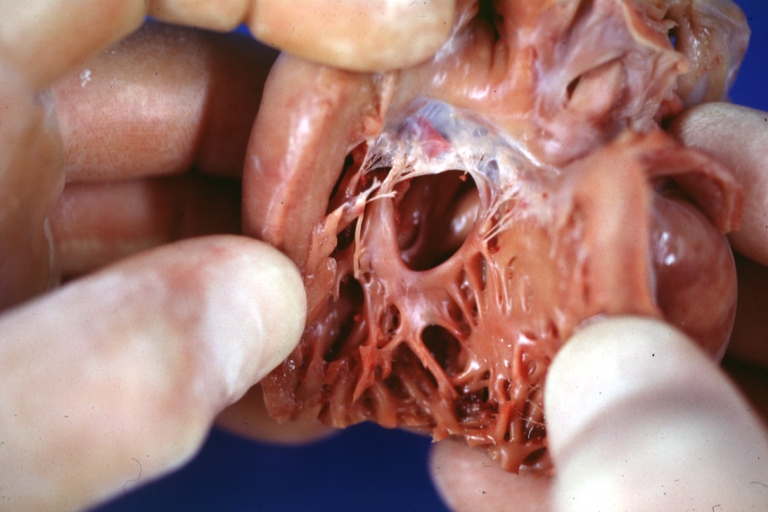
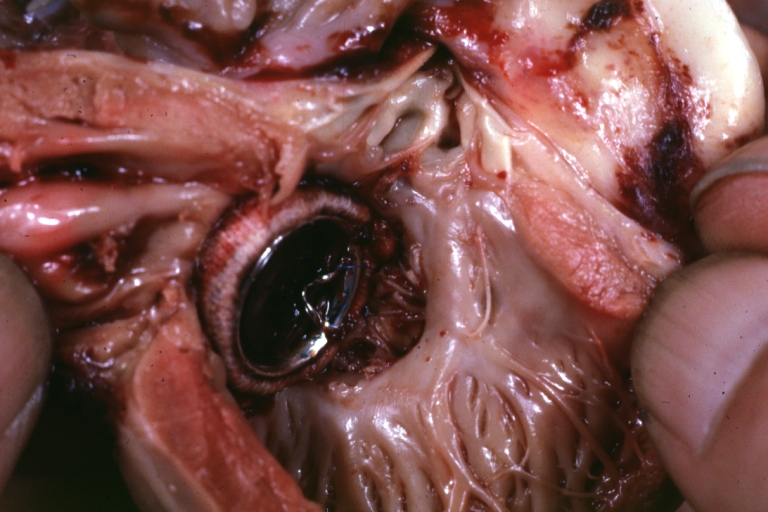
Interventricular Septal Defect (Muscular Septum): Gross, natural color, muscular septal defect in newborn
-
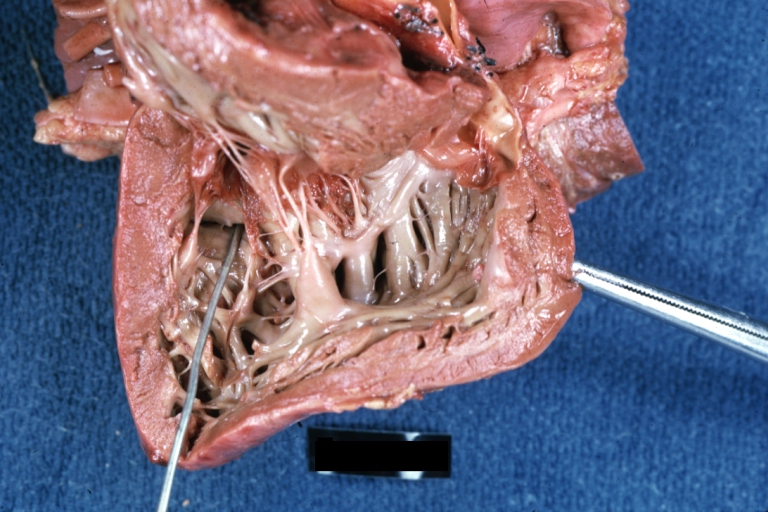
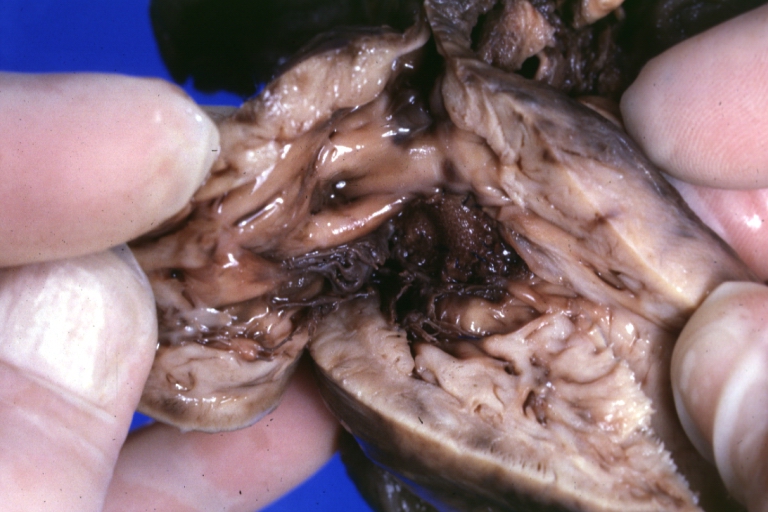
Subvalvular Ventricular Septal Defect: Gross, good view of defect with overriding aorta
-
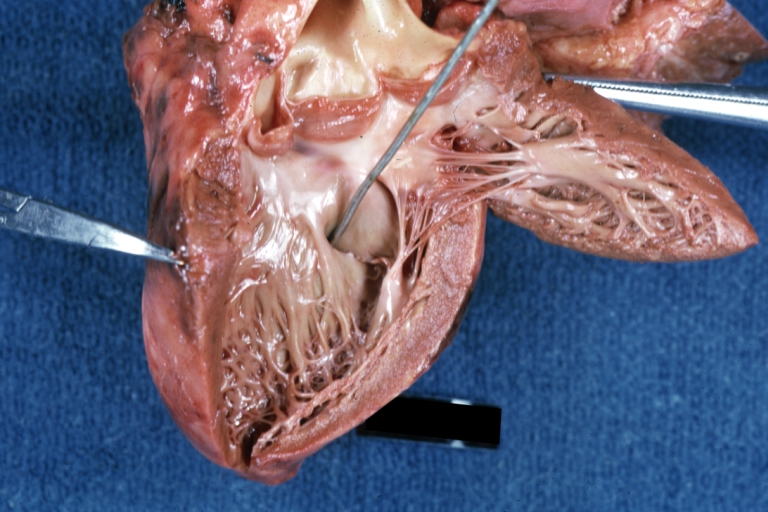
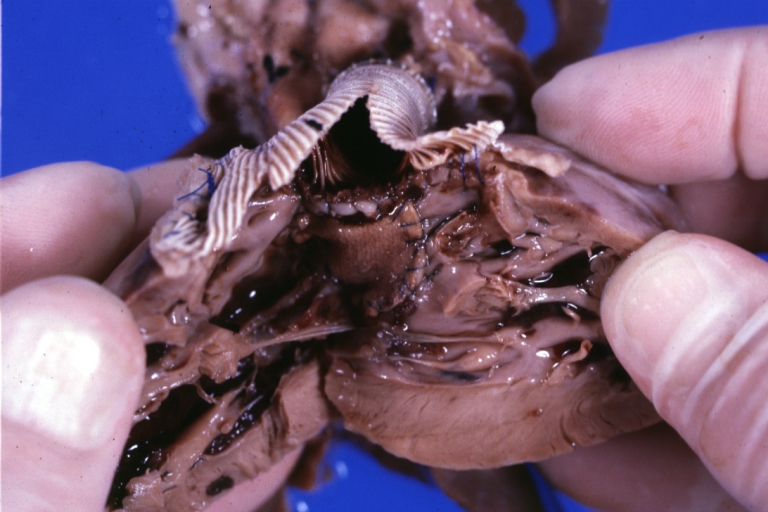
Ventricular Septal Defect: Gross, infant heart, pulmonary outlet, muscular septal defect
-
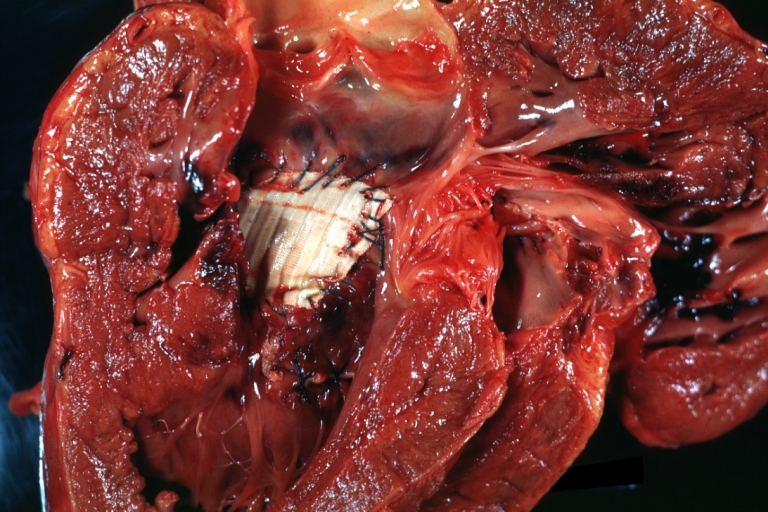
Perimembranous Ventricular Septal Defect: Gross, fixed tissue, lesion seen from right ventricle (with patch)
-
Perimembranous Interventricular Septal Defect: Gross, fixed tissue, view from right atrium and ventricle with patch placed three days prior to death.
-
Perimembranous Ventricular Septal Defect: Gross, an excellent example
-
Ventricular Septal Defect: Gross, subvalvular defect, left ventricle view of tetralogy of Fallot (very good example)
-
Ventricular septal defect
-
Subpulmonic Ventricular Septal Defect: Gross, a well shown lesion.
-
Subvalvular Ventricular Septal Defect
-
Subvalvular Ventricular Septal Defect
-
Ventricular Septal Defect: Gross, natural color, view of opened heart with lungs attached shows rather well a subvalvular VSD
-
Double Outlet Right Ventricle: Gross, fixed tissue, close-up view of left ventricular outflow tract and patched ventricular septal defect. The override is obvious in this (very good) close-up view
-
Perimembranous Ventricular Septal Defect: Gross, fixed tissue, opened left ventricular outflow tract into aorta. Defect was patched 3 days prior to death
-
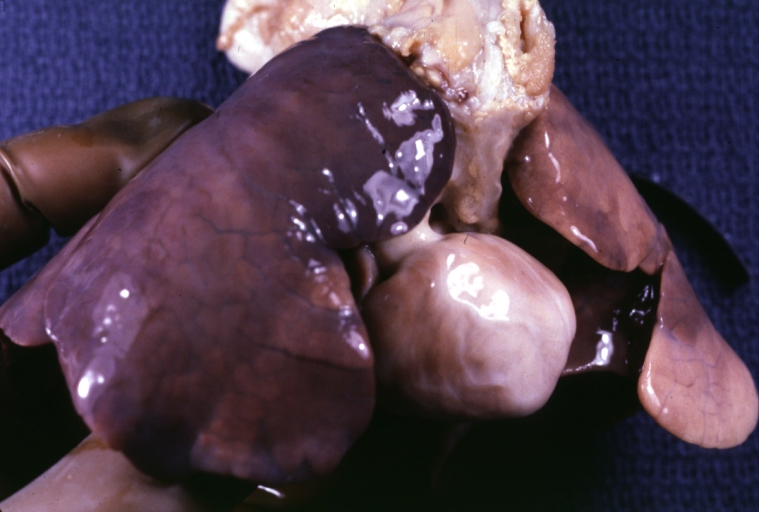
Atrial Septal Defect: Gross, (an excellent example) foramen ovale defect with right ventricular hypertrophy and fatty infiltration of the right ventricular wall, enlarged right atrium
-
Ventricular Septal Defect: Gross close-up adult heart, small perimembranous septal defect (very good example)
-
Interventricular Septal Defect (Muscular Septum): Gross, natural color, low septal defect shown from aortic outlet. The same defect (with a probe in hole) shown from right ventricle.
-
Interventricular Septal Defect (Muscular Septum): Gross natural color right ventricular outlet (probe in defect) view from left ventricular side
-
Atrial Septal Defect: Gross natural color infant heart foramen ovale defect, septum secundum
-
Aortic Subvalvular Ventricular Septal Defect: Gross, natural color, septal defect has patch repair. Aortic valve is myxomatous. A complex case of truncus with interrupted arch.
-
Interventricular Septal Defect Membranous Septum: Gross natural color close-up (an excellent demonstration)
-
Interventricular Septal Defect Membranous Septum: Gross natural color small defect well shown. Aortic cusps are scarred and one is perforated
-
Subvalvular Ventricular Septal Defect: Gross, natural color, close-up view of aortic outflow tract with a large subvalvular defect
-
Membranous Interventricular Septal Defect: Gross natural color subvalvular defect with probe immediately inferior to membranous septum
-
Subvalvular Ventricular Septal Defect: Gross, fixed tissue, large subpulmonic defect apparently represent left displacement of the pulmonary artery
-
Interventricular Septal Defect: Gross, fixed tissue, opened right ventricular outflow tract positioned to show perimembranous septal defect (as surgeon would see it during repair)
-
Ventricular Septal Defect Muscular: Gross, natural color, view from right ventricle with probe in defect right ventricular hypertrophy is evident
-
Ventricular Septal Defect Muscular: Gross, natural color, view from left ventricle with probe in defect
-
Interventricular Septal Defect Subvalvular with Patch Repair: Gross natural color 19yo with Tetralogy of Fallot also shows overriding aorta
-
Interventricular Septal Defect Subvalvular with Patch Repair: Gross, natural color, close-up
-
Interventricular Septal Defect (Perimembranous) with Patch Repair: Gross, natural color, view from right ventricle. A case of inverted ventricles
-
Interventricular Septal Defect (Perimembranous) with Patch Repair: Gross, natural color, view from left ventricular outflow tract
-
Ventricular Septal Defect (Subvalvular): Gross, fixed tissue, small heart with opened aorta and subvalvular defect shown. A case of pulmonary artery atresia
-
Truncus Arteriosus with Subvalvular Ventricular Septal Defect: Gross, natural color, an excellent view of subvalvular defect. Quadricuspid truncus valve and type I origin of pulmonary arteries
-
Truncus Arteriosus with Subvalvular Interventricular Septal Defect: Gross, natural color, defect is shown from the right side (view toward right ventricular outlet)
-
Truncus Arteriosus with Subvalvular Interventricular Septal Defect: Gross natural color excellent view of lesion looking at opened aortic ring with quadricuspid aortic valve. A large subvalvular defect (origin of pulmonary arteries is at forceps)
-
Av Canal with Left Side Bjork Shiley Prosthetic Valve: Gross, natural color, a close-up view of valve and the bridging defect
-
Interventricular Septal Defect (Perimembranous) with Patch Repair: Gross, fixed tissue, a close-up view of patch repair from right ventricle
-
Conduit Right Ventricle to Pulmonary Artery: Gross, fixed tissue, opened conduit showing sutures into ventricle and patch closed perimembranous interventricular septal defect
-
Ventricular Septal Defect (Perimembranous): Gross, natural color, (quite good photo - lesion before the operation)
-
Ventricular Septal Defect (Subvalvular) Repaired: Tetralogy of Fallot; Gross, fixed tissue, close-up view of a large subvalvular defect repaired with a Dacron patch (overgrown with fibrous tissue prominent subaortic shelf with endocardial thickening).
-
Ventricular Septal Defect (Subvalvular) Repaired: Tetralogy of Fallot; Gross, fixed tissue, close-up view of a large subvalvular defect repaired with a Dacron patch
-
Ventricular Septal Defect (Subvalvular) Repaired: Gross, fixed tissue, close-up view of Dacron patch. Nearly completely covered with fibrous tissue
-
Transposition Great Vessels with Interventricular Septal Defect: Gross, fixed tissue, opened left ventricular outflow tract into a pulmonary artery (perimembranous defect)
-
Transposition Great Vessels with Interventricular Septal Defect: Gross, fixed tissue, close-up of interventricular septal defect and pulmonary valve
References
- ↑ Anderson RH, Sarwark AE, Spicer DE, Backer CL (2014). "Exercises in anatomy: holes between the ventricles". Multimed Man Cardiothorac Surg. 2014. doi:10.1093/mmcts/mmu026. PMID 25547619.
- ↑ Anderson RH, Webb S, Brown NA, Lamers W, Moorman A (2003). "Development of the heart: (2) Septation of the atriums and ventricles". Heart. 89 (8): 949–58. PMC 1767797. PMID 12860885.
- ↑ Schleich JM, Abdulla T, Summers R, Houyel L (2013). "An overview of cardiac morphogenesis". Arch Cardiovasc Dis. 106 (11): 612–23. doi:10.1016/j.acvd.2013.07.001. PMID 24138816.
- ↑ 4.0 4.1 4.2 Anderson RH, Spicer DE, Brown NA, Mohun TJ (2014). "The development of septation in the four-chambered heart". Anat Rec (Hoboken). 297 (8): 1414–29. doi:10.1002/ar.22949. PMID 24863187.
- ↑ 5.0 5.1 Gittenberger-de Groot AC, Calkoen EE, Poelmann RE, Bartelings MM, Jongbloed MR (2014). "Morphogenesis and molecular considerations on congenital cardiac septal defects". Ann Med. 46 (8): 640–52. doi:10.3109/07853890.2014.959557. PMID 25307363.