Artificial pacemaker

Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2] Javaria Anwer M.D.[3]
Synonyms and keywords: artificial cardiac pacemaker, artificial heart pacemaker.
Overview
A pacemaker (or artificial pacemaker, not to be confused with the heart's natural pacemaker) is an electronic device which is used to treat cardiac arrhythmias. The device placed in the chest or abdomen uses electrical impulses, delivered by electrodes contacting the heart muscles to regulate the beating of the heart. The primary purpose of a pacemaker is to maintain an adequate heart rate, either because the heart's native pacemaker is not fast enough, or there is a block in the heart's electrical conduction system. Modern pacemakers are externally programmable and allow the cardiologist to select the optimum pacing modes for individual patients. Some combine a pacemaker and implantable defibrillator in a single implantable device. Others have multiple electrodes stimulating differing positions within the heart to improve synchronization of the lower chambers of the heart.
History of the Artificial Pacemaker
The story of artificial pacemaker development spans over a century with efforts from scientists all over the world.
- Artificial electric stimulation of the heart: It was long after the concept of heart block from Chinese physician Pien Ch’io that the treatment was sought, due to the scarce knowledge of the details of the cardiac conduction.[1] In 1775, the Danish physicist Nickolev Abildgaard revived a dead hen by applying the same electrodes over its chest, which when applied over its head, would cause death with the electric discharge. In 1788 Charles Kite attempted reported the successful attempt of using electric shock to revive a three-year old girl.[2] In 1889 J A McWilliam reported his experiments of application of an electrical impulse to the human heart in asystole to cause a ventricular contraction and that a heart rhythm of 60-70 beats per minute could be evoked by impulses applied at spacings equal to 60-70/minute.[3]
- Defibrillation: In 1926 Dr. Mark C Lidwill devised a portable apparatus with "one pole applied to a skin pad soaked in strong salt solution" and another pole "consisting of a needle insulated except at its point and was plunged into the appropriate cardiac chamber". The rate varied from about 80 to 120 pulses per minute, voltage variable from 1.5V to 120V. In 1928, the device was used to revive a stillborn infant at Crown Street Women's Hospital, Sydney after 10 minutes of stimulation.[4][5] In 1932 Albert Hyman described a hand-cranked motor-powered electro-mechanical instrument named as "artificial pacemaker", the term we continue to use till date.[6][7] Hymen (effected Lidwill's work too) could not publish his work on the use of his invented pacemaker due to the public perception of interfering with nature by 'reviving the dead'.[5]
- Transcutaneous pacemaker: In 1950 a Canadian electrical engineer John Hopps after observing a cardiothoracic surgeon, developed the first transcutaneous pacing device powered by AC current. The device made by using vacuum tube technology was painful to the patient with the hazard of electrocution. He also designed the first catheter-electrode for cardiac stimulation. Paul Zoll developed a bulky transcutaneous pacemaker with a rechargeable battery.[8] External pacing was painful and caused burns. In 1957 Dr. William L. Weirich's publication demonstrated the restoration of heart rate, cardiac output, and mean aortic pressures in animals with complete heart block through the use of a myocardial electrode.[9]
- Battery-operated pacemaker: The prevalence of post-operative heart block and complications associated with transcutaneous pacemakers led Lillehei and his co-workers to develop a Teflon sleeved stainless steel wire. The myocardial wire was implanted directly into the myocardium with the other end connected to an exterior stimulator. A remote electrode was buried under the skin. A 3-year-old girl was the first successful recipient of the myocardial wire. In 1957, a pacemaker power failure leading to the death of a baby prompted Earl Bakken, an electrical engineer design the first battery-operated pacemaker the same year. The availability of transistors in 1956 made a battery-operated device possible.
- Implantable pacemaker: In 1958, Rune Elmqvist and Åke Senningt's designed first implantable pacemaker was implanted at the Karolinska University Hospital in Sweden. Two electrodes were implanted into the myocardium and the pulse generator was placed in the abdominal wall. The pacemaker used silicone transistor and rechargeable nickel-cadmium batteries charged by an induction coil from the outside. The device failed after three hours and a second device was then implanted which lasted for two days. The world's first implantable pacemaker patient, Arne Larsson, survived the first tests and died in 2001 after having received 22 different pacemakers during his lifetime. In 1960, an improved version of the Elmqvist design was implanted in Uruguay by doctors Fiandra and Rubio. That device lasted until the patient died of other ailments, 9 months later.
- Transvenous pacemaker: In 1959 temporary transvenous pacing was first demonstrated by Furman et al in which the catheter electrode was inserted via the patient's basilic vein.[10] The first use of transvenous pacing in conjunction with an implanted pacemaker was by Parsonnet in the USA [11][12], Lageren in Sweden[13][14] and Jean-Jaques Welti in France[15] in 1962-63. The transvenous approach involved incision of a vein and insertion of catheter electrode lead under fluoroscopic guidance, until it was lodged within the trabeculae of the right ventricle. The method was the approach of choice the mid-1960s.
- Lithium battery: In 1960 implantable pacemakers constructed by engineer Wilson Greatbatch utilizing mercury-zinc battery as an energy source was used in humans from following extensive animal testing. The first patient lived for a further 18 months. Unreliability and short lifetime of the implantable devices utilizing mainly mercury battery led us to now available lithium batteries. Greatbatch invented the lithium–iodine battery, which powers the modern pacemakers.
- Improved leads: While the battery life was being improved, the unreliability of myocardial leads for longterm use was still an issue. The wires could not resist the mechanical stress, required an increasing capture threshold unless an exit block developed. In 1962, transvenous leads replaced myocardial leads and in the early 1980s, steroid-eluting leads ensured a decrease in the lead tip evoked inflammatory response. These techniques assured lead safety and resolved the capture threshold issue.
- Improved casing: A further impediment to the reliability of the early devices was the diffusion of water vapor from the body fluids through the epoxy resin encapsulation affecting the electronic circuitry. This phenomenon was overcome by encasing the pacemaker generator in a hermetically sealed metal case, initially by Telectronics of Australia in 1969 followed by Cardiac Pacemakers Inc of Minneapolis in 1972. This technology, using titanium as the encasing metal, became the standard by the mid-1970s.
- ICD: The first ICD device was implanted in 1980, by Levi Watkins at Johns Hopkins Hospital, after 11 years of research work. The patient survived for 10 years and died from unrelated causes. Initially, defibrillator patches were applied directly to the epicardium or pericardium via surgical thoracotomy. The device was placed in a subcutaneous abdominal wall pocket. Nowadays transvenous implantation technique is used, with the devices placed in below collar bone.[2]
- Leadless pacemaker: In 2013, multiple firms first announced of pill-sized pacemaker devices that could be inserted via a leg catheter.[16] The Nanostim pacemaker received CE marking in 2013. In November 2014 a patient, Bill Pike received a Medtronic Micra pacemaker in Providence St Vincent Hospital in Portland Oregon. In 2014 also St. Jude Medical Inc. announced the first enrollments in the company’s leadless Pacemaker Observational Study evaluating the Nanostim leadless pacing technology and post-approval implants have occurred in Europe.[17] The European study was recently stopped after there were reports of six perforations that led to two patient deaths. In the United States this therapy is still not approved by the FDA.[18] Single and dua-chamber leadless pacemakers are now available.[19]
- Reusable pacemaker: To avoid explosions, pacemakers have to be removed postmortem from bodies and thousands of pacemakers are removed by funeral home personnel each year all over the world. Since the 1970s, studies all over the world have reported on the safety and efficacy of pacemaker reuse. No widely acceptable standards for safe pacemaker and ICD reuse have been developed. [20] According to the National Academy of Medicine, in LMICs inadequate access to advanced cardiovascular technologies is one of the major contributors to cardiovascular disease morbidity and mortality.
Epidemiology and Demographics
Steady growth has been demonstrated in the market of pacemakers in the US and worldwide. It is estimated that people with an implantable pacemaker in the US range from 500,000 and 3 million. By 2023 the number of pacemakers globally is expected to increase to 1.43 million units. Between 1993 and 2009, 2.9 million patients received permanent pacemakers in the United States ( 55.6% increase in use).[21] Dual-chamber pacemaker (DDD) use increased by 20% and the use of single-chamber ventricular (VVI)pacemaker decreased.
- Age: Over 70% of all pacemakers are implanted in patients over the age of 65. The age of dual-chamber pacemaker recipients ranges from 73 to 75 years and the chance of getting a pacemaker dramatically increases with age.[21]
- Gender: Between 2006- 2016, both males and females equally choose DDD mode of pacing over VVI. Male patients had more CRT implantation.[22] In patients with sick sinus syndrome, young age, female gender, and non-Caucasian race were associated with a lower likelihood of pacemaker implantation.[23]
Indications
The indications are for both temporary and permanent pacing as temporary pacing is usually used as a bridge to permanent pacing. According to the jointly published guidelines from American College of Cardiology(ACC), American Heart Association(AHA), and the Heart Rhythm Society (HRS), the indications for pacemaker implantation have been classified into three categories based on the strength of recommendation. Class I: strong, Class IIa: moderate, Class IIb: weak, Class III: no benefit/ harm.[24] We describe the indications in class I (strongly recommended) below.
- Sinus node dysfunction (when the sinoatrial node does not fire properly to contract the heart) - the permanent pacing recommendation guideline does not have a fixed minimum heart rate or pause duration.
- Heart block - Chronic Bifascicular block, trifascicular block, or third degree AV block.
- Post acute myocardial infarction[25]
- Hypersensitive carotid sinus syndrome and cardioinhibitory vasovagal syncope - can be considered in patients 40 years or older with recurrent syncope and associated asystole.[26][27] Dual-chamber pacing with closed-loop system sensing has been shown to be effective due to the earlier outset of pacing. [28][29]
- Congenital heart disease - used to prevent sudden cardiac death, improve the quality of life of the patients and as a bridge to heart transplant.[30]
- Pacing to prevent tachycardia
- Post heart transplantation
Someother indications include:
- Stokes-Adams attack involving disruption of conduction between the sinoatrial node and the atrioventricular node.
- Heart failure [31]
- Hypertrophic cardiomyopathy[32] - Biventricular pacing has demonstrated improvement in functional capacity.[33]
- Long QT Syndrome[34]
Contraindications for pacing
Class III of the jointly published guidelines from ACC, AHA, HRS described certain conditions where pacing can be harmful/ contraindicated. They include asymptomatic sinus bradycardia or second-degree Mobitz type-I block, Long QT due to reversible causes, presence of accessory pathway, to name a few.[24] Risks and benefits are weighed for every patient and vary on a case by case basis. A few general relative contraindications to pacing include: Bacteremia or local infection at the implant site, large ventricular infarct, excessive bleeding risk, hemorrhagic diathesis and PEEP ventilation.
Methods of Pacing
Cardiac pacemakers consist of two parts: a pulse generator or simply generator which is the source of electric pulse, and a variable number of leads that convey the electric signal from the generator to the myocardium. The pacemaker generator is an airtight sealed device containing a power source, usually a lithium battery. The sensing amplifier processes the electrical manifestation of naturally occurring heartbeats as sensed by the heart electrodes, the computer logic for the pacemaker, and the output circuitry which delivers the pacing impulse to the electrodes. Newer leadless pacemakers have been introduced reducing the risk of complications. There is no formal classification system for the pacemaker. Based upon their duration of use they can be divided into temporary pacing or emergency use pacing and permanent pacing.
Temporary pacing
Temporary pacing is indicated if permanent pacing is not instantly available, not required, or contraindicated. Unlike a permanent pacemaker, the generator is placed outside the body and not implanted in the subcutaneous tissue. Depending upon the use temporary pacing for single chamber or dual chamber pacing. Different types of temporary pacing techniques (based upon the approach used to consign the leads to the heart chambers) have been described in the table below.
| Types of temporary pacing | |||
|---|---|---|---|
| Type | Procedure | Use | Complication/ Limitation |
| Temporary Transvenous pacing | Under sterile conditions, a pacemaker wire guided by fluoroscopy or echocardiography is placed into a vein. Femoral vein/ Internal jugular vein or subclavian vein are the most common access sites.[35] It is then passed into either the right atrium or right ventricle. The pacing wire is then connected to an external pacemaker outside the body and appropriate mode is selected. | 1. Atrioventricular block[36] 2. Alternative to transcutaneous pacing and a bridge to permanent pacing. 3. Post-op injury/ trauma or temporary damage to conduction system or SA node. |
1. Infection 2.Lead dislodgment and malfunction 3. Thrombosis 4. Pneumothorax, hemothorax 5. Pericardial tamponade[37] |
| Transcutaneous pacing/ External pacing | The procedure described in: External pacing should not be relied upon for an extended period of time. | 1. Hemodynamically significant bradycardias, asystole, third-degree AV block[38] 2. bridge to transvenous pacing |
1. Patient discomfort 2. Risk of skin burns 3. Musculoskeletal stimulation |
| Epicardial Pacing | Temporary epicardial leads are placed towards the end of an open-heart surgery. The electrodes are placed in contact with the outer wall of the right ventricle or the right atrium also, to maintain satisfactory cardiac output. The procedure needs care and only battery-powered equipment can be used to prevent damage from AC current.[39] | 1. Differentiate junctional tachycardia from SVT( Atrial electrogram) and degrees of heart block 2. Reentrant tachycardia - atrial flutter, SVT.[39] |
1. Infection 2. Arrhythmias 3. Myocardial damage 4.Cardiac temponade |
| Transesophageal pacing | Two types of leads are available; pill electrode with flexible wire that can be swallowed and a flexible catheter that is introduced via nares under local anesthesia. Transesophageal pacing requires special pacing devices. Being programed for burst pacing only, esophageal stimulators are intrinsically developed as triggering systems. The addition of a programmable stimulator is required to deliver extrastilumi. | 1. Evaluation of SA node, supraventricular tachycardias and palpitations. 2. Atrial fibrillation[40] 3. Replacement of invasive atrial pacing (Atrial proximity and safer technique) |
1. No ventricular pacing 2. Heartburn 3. Phrenic nerve stimulation[41] |
| Transthoracic pacing | The old technique involves utilising a needle trocar to introduce the electrode into the ventricle percutaneously. The technique was introduced as an emergency measure when no other techniques were available. With better techniques available these days, transthoracic pacing is no longer used. | Only in a hemodynamically unstable patient where other pacing techniques are unavailable. | 1. Painful procedure 2. Injury to vital organs |
| Transthoracic mechanical pacing | An old procedure, also known as percussive pacing involves the use of the closed fist, usually on the left lower edge of the sternum over the right ventricle. According to the British Journal of Anesthesia Striking from a distance of 20 - 30 cm to raise the ventricular pressure 10 - 15mmHg will induce electrical activity and hence ventricular beat.[42] It is a life-saving mean until an electrical pacemaker is available. | 1.Bradyarrhythmias with hemodynamic instability or asystole[43] 2. Complete heart block with ventricular asystole |
1. Potential for injury due to mechanical nature |
| Transmediastinal pacing | The approach is old but can also be utilized for permanent pacing. In general, under general anesthesia, the procedure involves costal cartilage resection, the reflection of pleura, and insertion of epicardial electrodes below the epicardium. The electrode leads passes beneath the xiphoid and is attached to the pulse generator in the left lower quadrant. | Atrial pacing | 1. Intraoperative complications, hemorrhage 2. Lead displacement 3. Infection |
Permanent Pacing
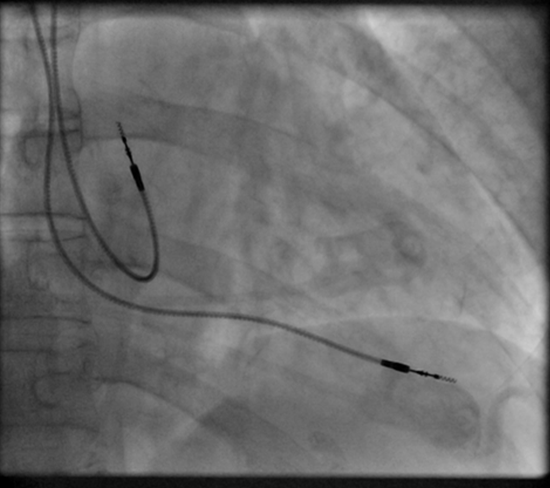
Permanent pacing achieved with an implantable pacemaker usually involves the transvenous placement of one or more pacing electrodes within a chamber, or chambers, of the heart. The access sites are usually subclavian vein, internal jugular vein or femoral vein. A case of transhepatic venous access has also been reported.[44] The electrode lead is passed along the vein, through the valve of the heart, until positioned in the chamber. The procedure is facilitated by fluoroscopy which enables the physician or cardiologist to view the passage of the electrode lead. After satisfactory lodgment of the electrode is confirmed, the opposite end of the electrode lead is connected to the pacemaker generator. Most commonly, the generator is placed below the subcutaneous fat of the chest wall, above the muscles and bones of the chest. However, the placement may vary on a case by case basis. In the pediatric population, intraabdominal/ subperitoneal placement of the generator has been reported. The outer casing of pacemakers made up of titanium to limit the rejection by the body's immune system.
Types of Pacemakers
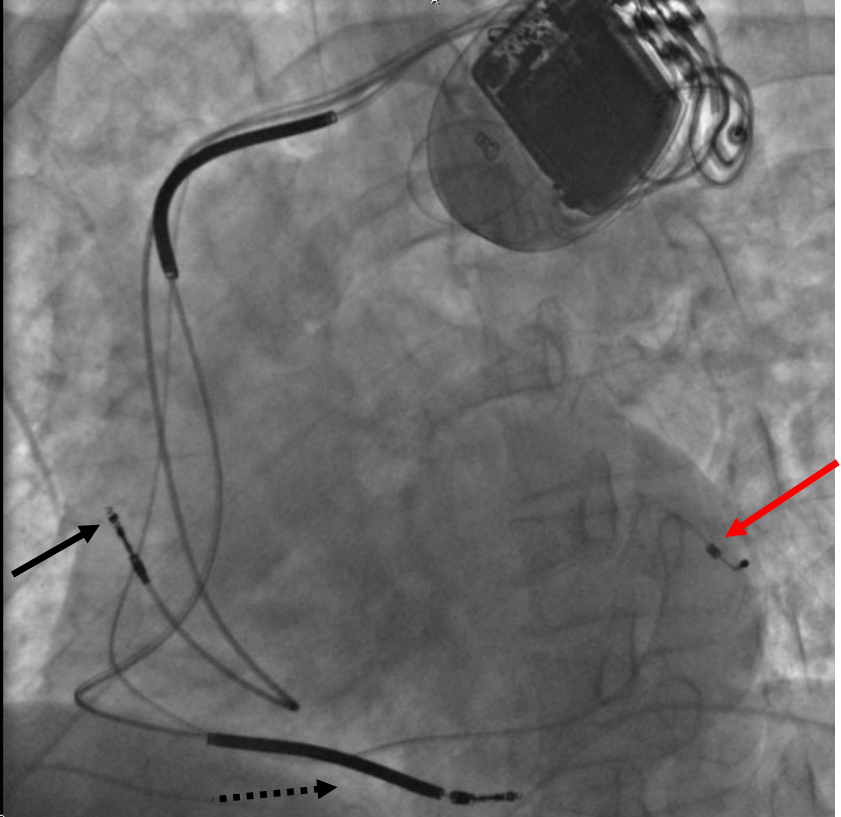
Based on the number of chambers involved, a pacemaker can be divided into three types: single-chamber, dual-chamber, and biventricular pacemaker. Based upon the mechanism of rate control used by the pacemaker and new advancements in rate-control function, pacemakers can be divided into fixed-rate versus rate-responsive pacemakers.
Single-chamber pacemaker
The generator is connected to a single lead that is used to pace one chamber of the heart, either the right atrium or right ventricle.
Leadless pacemaker
The leadless pacemaker developed over the last few years is a new device that is smaller than the conventional pacemakers. An FDA approved leadless pacemaker "Micra" is 25.9 × 6.7 mm in dimensions. The need for leads is eliminated as the device contains the generator and electrodes and is implanted directly in the ventricle via femoral vein. It prevents the complications associated with the widely used transvenous pacing, such as lead dislodgment and infection. As a limitation, the device is a single-chamber pacemaker and is unable to defibrillate.[45]
Dual-chamber pacemaker
In dual-chamber pacing (DDD) the generator is connected to two leads that are used to pace both right atrium and right ventricle at the same time. The pacemaker is regulated to pace both chambers in a regular fashion ensuring the optimal function of the chambers. Although dual-chamber models are usually more expensive, the ability to coordinate the contractions of the atria to precede that of the ventricles can be useful in congestive heart failure. The DAVID trials[46] have shown that unnecessary pacing of the right ventricle can lead to heart failure and an increased incidence of atrial fibrillation. Dual-chamber devices can keep the amount of right ventricle pacing to a minimum and prevent the worsening of heart disease.
Biventricular pacemaker
A biventricular pacemaker (BVP or BiV), also known as CRT (cardiac resynchronization therapy) consists of three leads, one in the right ventricle to stimulate the septum, and another inserted through the coronary sinus to pace the lateral wall of the left ventricle and for patients in normal sinus rhythm, there is a third lead in the right atrium to facilitate synchrony with the atrial contraction.
Left ventricular dyssynchrony (an unsynchronized contraction of the right and left ventricles), an important cause of heart failure is observed in 25-50% of heart failure patients. CRT therapy, via its pacing function on both the septal and lateral walls of the left ventricle, can resynchronize the heart ensuring improved cardiac output.[47]
For patients with heart failure symptoms; a LVEF less than or equal to 35% and QRS duration of 120 msec or greater, CRT has been shown to reduce mortality and improve quality of life.[48][49][50] Biventricular pacing alone is referred to as CRT-P (for pacing). For selected patients at risk of arrhythmias, effective protection against the arrhythmias can be attained by combining CRT with an ICD, known as CRT-D (for defibrillation).[51][52]
Rate-responsive pacing
Rate-responsive pacing or rate-adaptive pacing is a relatively new technique. Traditional pacemakers worked on the principle of fixed predetermined heart rate or intermittent control. It limited the ability of artificial pacing to cater to the changing metabolic demands of the body. It led to the development of a "dynamic pacemaker" that could compensate for both actual respiratory loading and potentially anticipated respiratory loading. Scientists studied different bodily input parameters such as respiratory rate, minute ventilation, peak endocardial acceleration, central venous temperature, stroke volume, QT interval, venous pH, body motion, and changes of the right ventricular impedance during the cardiac cycle (CLS, closed-loop stimulation) to help make pacemakers responsive to body requirements.[53]
Rate-responsive or rate-adaptive pacing, allows the device to adjust the heart rate via rate response algorithms according to the body's metabolic requirements. By sensing the physical activity of the patient, rate-adaptive pacing can help optimize heart rate during activities such as exercise. The limitation to choose between sensitive or specific sensors has led the scientists to propose dual-sensor pacemakers and closed-loop stimulation pacing.[54] This dynamic pacemaking technology can also be applied to future artificial hearts.
His bundle pacing (HBP)
Studies have shown that traditional right ventricular apical pacing has been associated with heart failure (especially in patients with reduced LVEF)[55], arrhythmias, and potentially shorter life expectancy. Although benefits of CRT over right ventricular pacing (RVP) are established, CRT use is not established in patients with preserved EF.[56]
HBP is reported to be as effective as CRT.[57]
The technique is optimal for patients with bundle branch disease.
His bundle pacing technique stimulates the His–Purkinje fiber network directly with a special lead and placement technique, causing more effective ventricular activation. The two forms of His bundle capture by the pacing stimulus are selective capture (only His bundle tissue is captured), and non-selective capture (His bundle along with adjacent ventricular tissue is captured). His bundle pacing improves life expectancy and hospitalizations due to heart failure.[58]
With the advent of advancements in instrumentation, the variable anatomy of the bundles is not a limitation anymore and the average implant success rate of 84.8 % is achieved.[59] More research is required before HBP is considered a preferred modality for certain conduction disorders such as bundle branch blocks.
Basic Pacemaker Function
In a healthy heart SA node acts as the natural pacemaker by generating electrical impulses with automaticity. The impulses are then carried through AV node, bundle of His, bundle branches, and purkinje fibers to the ventricular myocardium. Regular and normal myocardial contractility is vital to hemodynamic stability. Any abnormality across the conduction path with symptomatic manifestations warrant the use of an artificial pacemaker. Modern pacemakers usually have multiple functions. The most basic form monitors the heart's native electrical rhythm. When the pacemaker doesn't sense a heartbeat within a normal beat-to-beat time period, it will stimulate the ventricle of the heart with a short low voltage pulse. This sensing and stimulating activity continue on a beat by beat basis.
The more complex forms include the ability to sense and/or stimulate both the atrial and ventricular chambers. Along with the fixed-rate pacemakers, rate-responsive pacemakers have been introduced as described above. From this the basic ventricular "on demand" pacing mode is VVI or with automatic rate adjustment for exercise VVIR – this mode is suitable when no synchronization with the atrial beat is required, as in atrial fibrillation. The equivalent atrial pacing mode is AAI or AAIR which is the mode of choice when atrioventricular conduction is intact but the natural pacemaker the sinoatrial node is unreliable – sinus node disease (SND) or sick sinus syndrome. Where the problem is atrioventricular block (AVB) the pacemaker is required to detect (sense) the atrial beat and after a normal delay (0.1–0.2 seconds) trigger a ventricular beat, unless it has already happened – this is VDD mode and can be achieved with a single pacing lead with electrodes in the right atrium (to sense) and ventricle (to sense and pace). These modes AAIR and VDD are unusual in the US but widely used in Latin America and Europe.[60][61] The DDDR mode is most commonly used as it covers all the options though the pacemakers require separate atrial and ventricular leads and are more complex, requiring careful programming of their functions for optimal results.
| I | II | III | IV | V |
|---|---|---|---|---|
| Chamber(s) paced | Chamber(s) sensed | Response to sensing | Rate modulation | Multisite pacing |
| O = None | O = None | O = None | O = None | O = None |
| A = Atrium | A = Atrium | T = Triggered | R = Rate modulation | A = Atrium |
| V = Ventricle | V = Ventricle | I = Inhibited | V = Ventricle | |
| D = Dual (A+V) | D = Dual (A+V) | D = Dual (T+I) | D = Dual (A+V) |
Other Devices with Pacemaker Function
Sometimes devices resembling pacemakers, called ICDs (implantable cardioverter-defibrillators) are implanted. These devices are often used in the treatment of patients at risk from sudden cardiac death. An ICD has the ability to treat many types of heart rhythm disturbances by means of pacing, cardioversion, or defibrillation. In patients with advanced heart failure with prolonged QRS, CRT combined with an implantable defibrillator has shown to significantly reduced mortality.[63]
| I | II | III | IV |
|---|---|---|---|
| Shock chamber | Antitachycardia pacing chamber | Tachycardia detection | Antibradycardia pacing chamber |
| O = None | O = None | E = Electrogram | O = None |
| A = Atrium | A = Atrium | H = Hemodynamic | A = Atrium |
| V = Ventricle | V = Ventricle | V = Ventricle | |
| D = Dual (A+V) | D = Dual (A+V) | D = Dual (A+V) |
| ICD-S | ICD with shock capability only |
| ICD-B | ICD with bradycardia pacing as well as shock |
| ICD-T | ICD with tachycardia (and bradycardia) pacing as well as shock |
Patient Considerations
Insertion
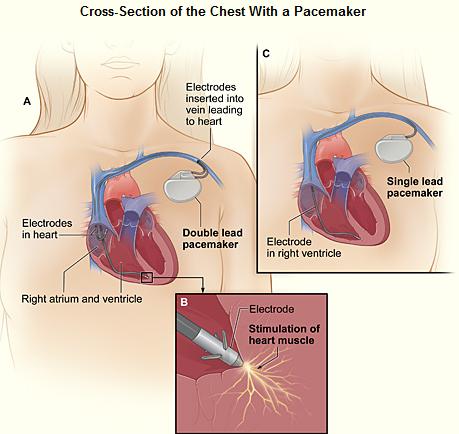
The patient may be given oral or intravenous antibiotics to prevent infection.[65]
Blood thinners and other heart medications may be stopped days before the procedure. The patients are required to stop eating midnight before and remove jewelry before the procedure. A drug is given for relaxation. Transvenous pacing procedure occurs in the pacemaker lab while an epicardial pacemaker implant may take place in an operating room. The patient is awake using a local anesthetic to numb the skin with or without sedation, or asleep using a general anesthetic.[66] The skin is shaved, cleaned, and draped. The monitoring devices attached to the patient/ available are ECG, pulse oximeter, blood pressure monitor, fluoroscope and defibrillator or pacemaker.
The lead or leads (the number of leads varies depending on the type of pacemaker) are fed into the heart through a large vein (approached through neck or groin) using a fluoroscope to monitor the progress of lead insertion. A pacemaker lead is implanted into the heart muscle with a miniature screw or anchored with small plastic hooks called tines. After the local anesthetic is given on the left or right shoulder (patient's non-dominant side selected), an incision is made below the collar bone to create a pocket created to contain the pacemaker generator. A temporary drain may be installed and removed the following day. The surgery may take 30-90 minutes. CXR is done to confirm the lead and pacemaker position and it is ensured that the pacemaker is properly functioning. Patients are often discharged on the same day. Wound-care is necessary to prevent complications.
The batteries within a pacemaker generator typically last 5 to 10 years. When the batteries are nearing the end of life, the generator is replaced in a procedure that is usually simpler than a new implant. Lead complications require lead replacement. Lead replacement mostly involves the removal of the already present leads and inserting replacements. Previous leads can be kept in place but they may hinder heart valve function or blood flow. Leads can normally be disconnected/ unhooked from the generator easily as happens in device replacement surgery. The possible complications, such as perforation of the heart wall, come from removing the lead{s} from the patient's body. Longer leads are more likely to have attachments to the patient's body in the pathway from device to the myocardium due to the body's defense mechanism of tissue encapsulation with time. So depending upon the chronicity of the lead implant, removal may involve simple traction or laser or cutting device to remove any organic attachments.
Pacemaker Patient Identification Card
International Pacemaker Patient Identification Cards carry information such as; patient data (between others, symptom primary, ECG, aetiology), pacemaker center (doctor, hospital), IPG (rate, mode, date of implantation, MFG, type) and lead [67] [68].
Living with a Pacemaker
Periodic Pacemaker Checkups
Once implanted, the pacemaker is periodically evaluated for function, patient safety, and troubleshooting problems. A complete pacemaker check is done six weeks after the implant and routine check is usually performed in-office every three or six months or depending on the frequency set due to patient or device factors such as battery life. For the pediatric population, a six month follow up is usually scheduled. The patients can forward their pacemaker data remotely via a mobile application. Patients with pacemakers also need to see a cardiologist at least once a year.
At the in-office follow-up, the physician can use a programmer/recorder/monitor (PRM) device to access pacemaker functions. Tests performed to assess the battery and function of the device include:
- Pacing lead Impedance[69]: A measure of lead integrity. Large and/or sudden increases in impedance can be indicative of a lead fracture while large and/or sudden decreases in impedance can signify a breach in lead insulation.
- Sensing: The ability of the device to "see" atrial and ventricular depolarization.
- Pacing threshold: It is the minimum amount of energy (both volts and pulse width) required to reliably depolarize (capture) the chamber being tested. It allows the medical personnel to program output with an appropriate safety margin and optimize device longevity.
- Events: Any important events such as extrasystoles, atrial or ventricular tachycardia such as atrial fibrillation that were stored since the last follow-up can be monitored. This is especially helpful in diagnosing the cause or origin of the event and making any necessary programming changes.
- Estimated battery life: With the advent of modern pacemakers "on-demand" pacemakers such as rate-responsive pacemakers, device longevity is affected by its utilization. Other factors such as programmed output and algorithms causing a higher-level of current utilization affect device longevity.
- Threshold duration: The amount of time that the device requires at the preset amplitude to reliably pace the atrium or ventricle connected to the lead.
- Percentage of pacing: The percentage of time that the pacemaker has been actively pacing since the previous follow-up (defines the extent of patient dependence on the device).
Lifestyle Considerations
Patients with a pacemaker lead a normal life requiring a few lifestyle modifications. For instance, if the shoulder harness of a vehicle seatbelt falls across the pacemaker insertion site, it may be uncomfortable for the patient. Patients are advised to refrain from collision sports or contact sports due to the potential for damage to the device or the heart condition itself.
Any kind of activity that involves intense magnetic fields should be avoided. This includes activities such as arc welding possibly, with certain types of equipment[70], or maintaining heavy equipment that may generate intense magnetic fields. FDA recommends avoiding placing a turned-on cellular phone next to the pacemaker and advises to maintain a possible distance from the device.[71] A 2008 U.S. study found[72] that the magnets in some portable music player headphones may interfere with pacemakers when placed in close proximity. Due to a recently reported case of magnetic reversion of an ICD, it is not advised to place an electronic cigarette close to a pacemaker or ICD.[73] Pacemakers and ICDs may set off airport metal detector alarms, but the metal detector gates have been demonstrated not to cause device malfuction. [74]
The patient should inform all medical personnel that the patient does have a pacemaker as certain investigations and procedures such as PET scan, Lithotripsy, MRI scan need considerations.[75] Newer pacemakers are MRI compatible. An MRI conditional device has to be reprogrammed right before and right after MRI scanning and certain patient qualifications need to be met to get an MRI compatible pacemaker. All the 5 most common cardiac pacing device manufacturers (covering more than 99% of the US market) now have FDA-approved MR-conditional pacemakers.[76]
Privacy and Security
Security and privacy concerns have been raised with pacemakers that allow wireless communication. A team of researchers demonstrated (at a short-range) that unauthorized third parties may be able to manipulate patient data in the pacemaker, or reprogram the devices[77] Researchers have developed a prototype firewall device (MedMon), designed to protect wireless medical devices such as pacemakers and insulin pumps from the attackers.[78]
Turning off a pacemaker
There is currently no legal precedent involving pacemakers in the United States of America. According to the Heart Rhythm Society (HRS), it was legal and ethical to honor requests by patients, or by those with legal authority to make decisions for patients, to deactivate implanted cardiac devices. Physicians have a right to refuse to turn it off, but are advised by the HRS panel that they should refer the patient to a physician who will.[79] Patient education and discussion regarding pacemaker devices at the end of life should be an important part of end-of-life discussions.[80]
Cost of pacemaker procedures
According to AHA 2019 Heart Disease and Stroke Statistics, a pacemaker costs $83,521, and ICD costs $171,476. The costs may vary depending upon patient condition, insurance, state, and other factors.
Complications of pacing
- Procedural: Although the pacemaker implant procedure has been associated with minimal complications. A few procedure-related complications that could occur include: Bruising/ bleeding or hematoma at the generator site (especially in elderly, chronic steroid users, if the patient is taking blood thinners)[81]. Cardiac chamber access issues, pericarditis, pneumothorax, hemothorax, valvular damage and cardiac tamponade are some other complications.
- Infection: Sepsis or infection at the implant site (rate is decreased with the use of periprocedural antibiotics) and infective endocarditis.
- Allergic reaction: Allergic reaction to the dye or anesthesia during the procedure and dermatitis from titanium or nickel sensitivity[82].
- Device malfunction: Failure to sense, capture or output. Sometimesdiaphragm stimulation can lead to ventilation problems.
- Conduction: Pacemaker syndrome, a phenomenon in which the patient presents with progressive worsening of symptoms due to low cardiac output after pacemaker implant. Patient may have dyspnea, syncope, hypotension or symptomatic bradycardia. It is due to atrioventricular dyssynchrony causing the ventricular origin of the pathway and loss of atrial contribution to the preload. Immediate treatment is supportive and long-term treatment involves altering the pacemaker to restore atrioventricular synchrony (changing the pacemaker from single-chamber to dual-chamber pacing or to dual-ventricular pacing). Bradycardia is a risk factor for the development of pacemaker syndrome.
A possible complication of dual-chamber artificial pacemakers is pacemaker-mediated tachycardia (PMT), a form of reentrant tachycardia. In PMT, the artificial pacemaker forms the anterograde (atrium to ventricle) limb of the circuit and the atrioventricular (AV) node forms the retrograde limb (ventricle to atrium) of the circuit.[83] Treatment of PMT typically involves reprogramming the pacemaker.[83] Another possible complication is pacemaker-tracked tachycardia, where a supraventricular tachycardia such as atrial fibrillation or atrial flutter is tracked by the pacemaker and produces beats from a ventricular lead. Newer devices are now programmed to recognize supraventricular tachycardias and switch to non-tracking modes, making this complication rare.
- Lead: Leads, with small diameter wires, can get infected or degrade due to lead flexing[84] requiring removal. The degradation problem can be overcome partly by certain changes to the programming of the pacemaker. However, leads reused for a longer period of time need replacement. Pacemaker lead malposition in various locations has been described in the literature and the location of the lead and symptoms determine the treatment.[85] Twiddler's syndrome results from patient manipulation of the pulse generator, causing the leads to be removed from their intended location and causing pacing failure or possible stimulation of other nerves. The management involves repositioning the leads and generator with suture fixation and patient counseling.[86]
Chest X Ray
-
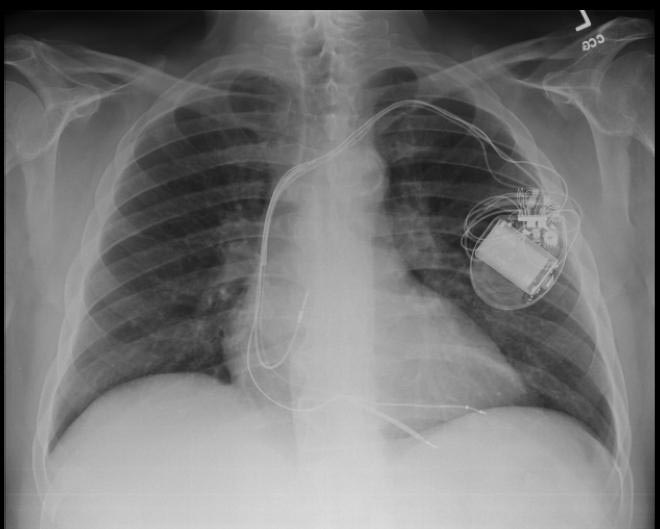
CXR showing Trilead cardiac pacer Image courtesy of RadsWiki and copylefted
-
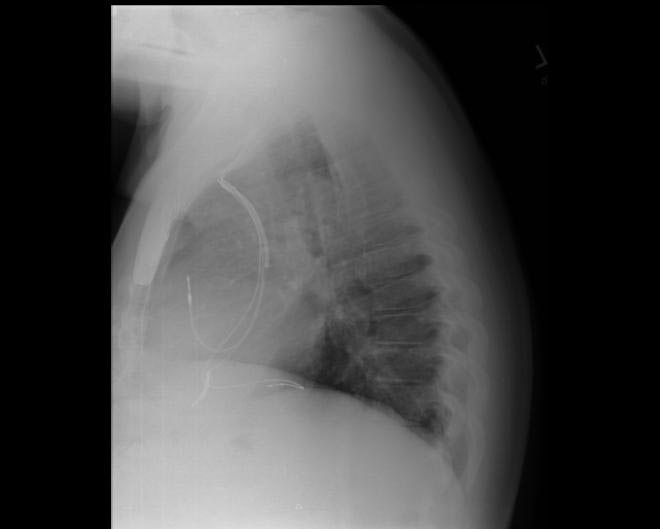
CXR showing Trilead cardiac pacer Image courtesy of RadsWiki and copylefted
-
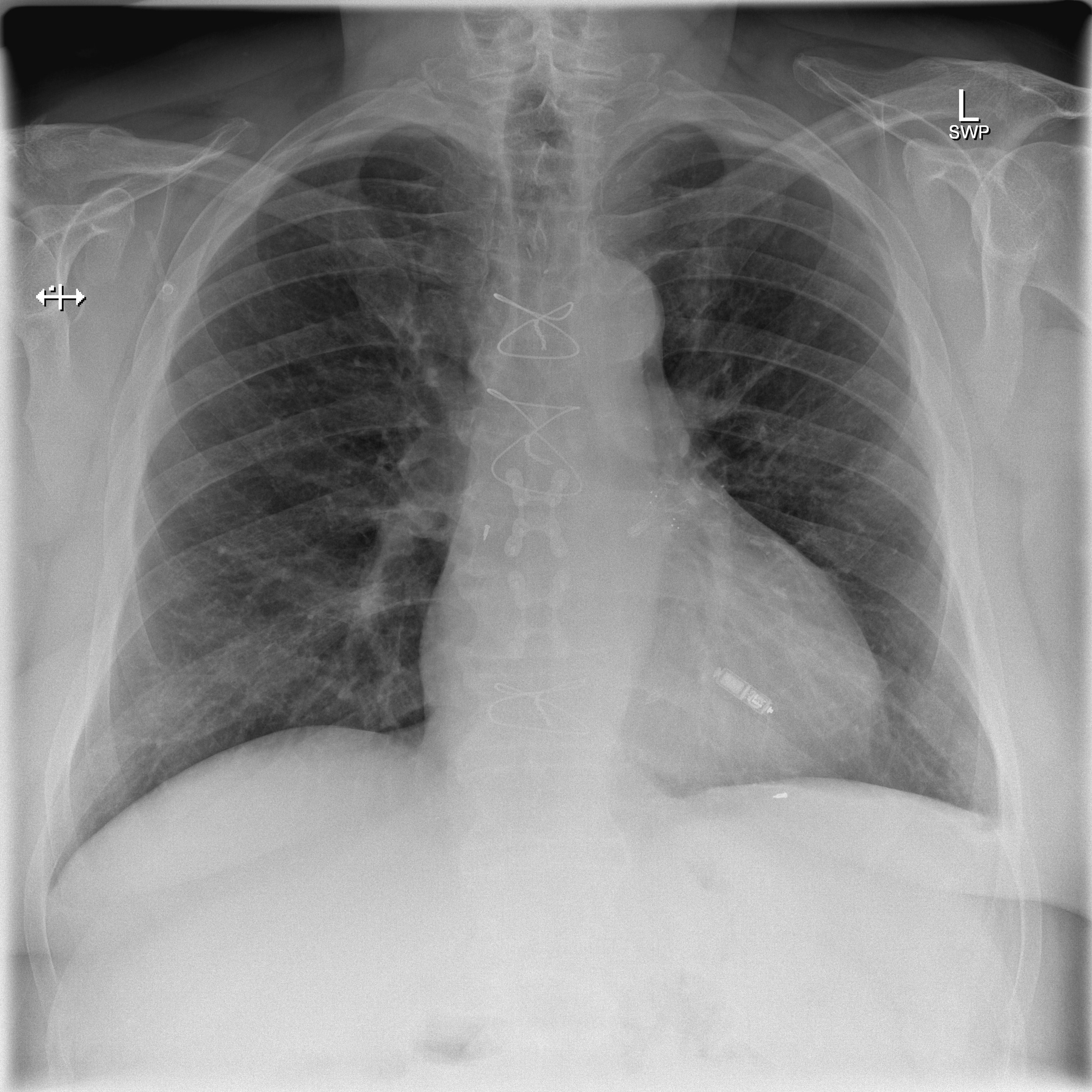
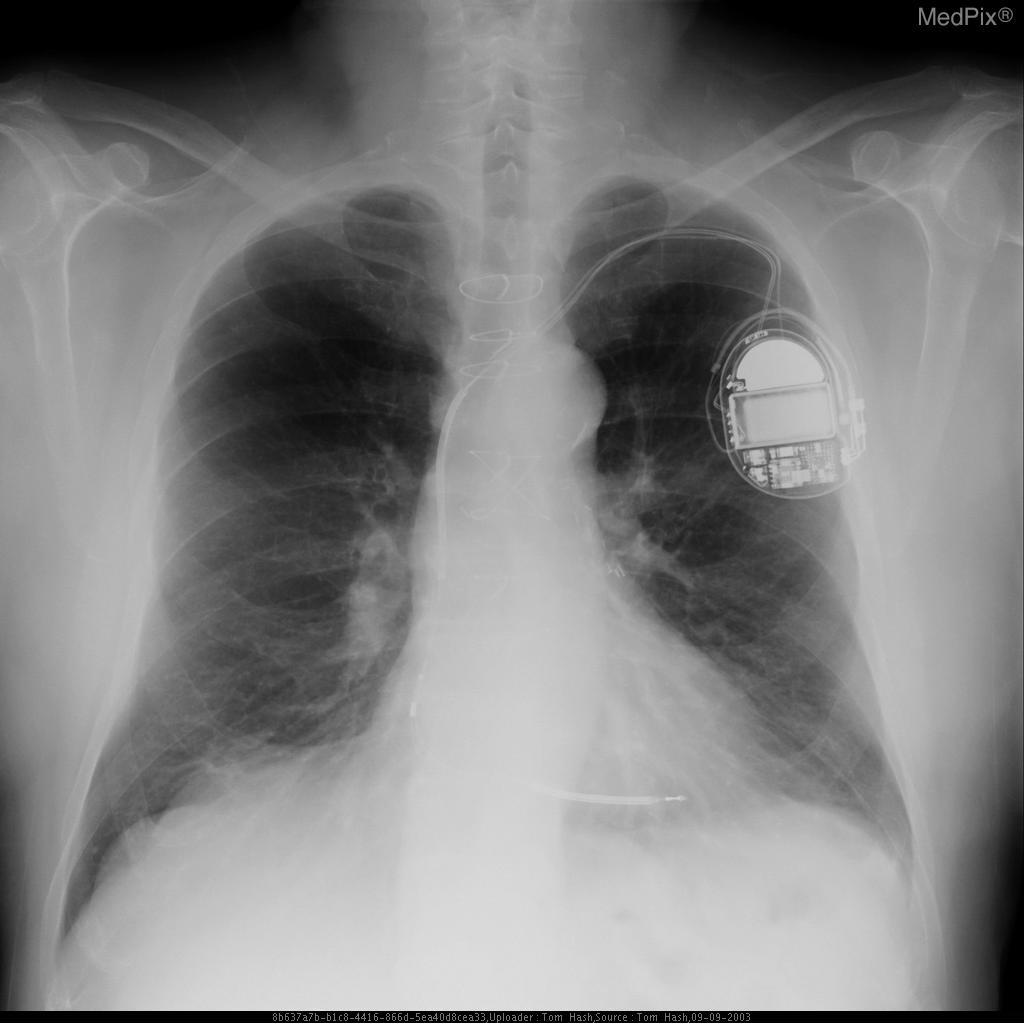
CXR showing a leadless pacemaker in the right ventricle, not to mistake the device with implantable loop recorder (ILR) - Case courtesy of Dr Marianne Cossens , Radiopaedia.org, rID: 61188
Electrocardiogram
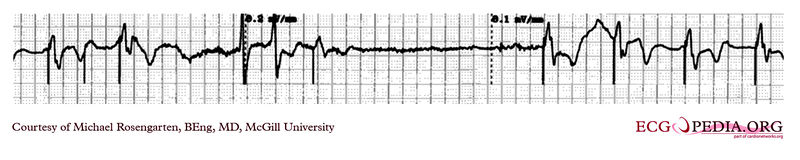
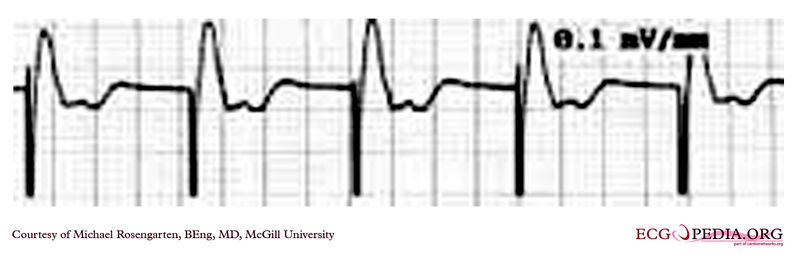
Shown below is an EKG demonstrating the determination of the atrial capture threshold. The pacemaker is decrementing down the atrial amplitude as marked below the atrial pacer spikes. Note the loss of atrial capture after the second spike of 1.5 volts. Loss of capture is illustrated by three events:
- 1.The lack of a P wave after the spike.
- 2.The appearance of a native P wave after the failure to capture.
- 3.The change in the RR intervals as the QRS is no longer following the paced P wave.
The lack of capture after the second 1.5 volt complex illustrates a component of time dependent capture where after a while a stimulus may fail to capture even thought the amplitude is kept constant.
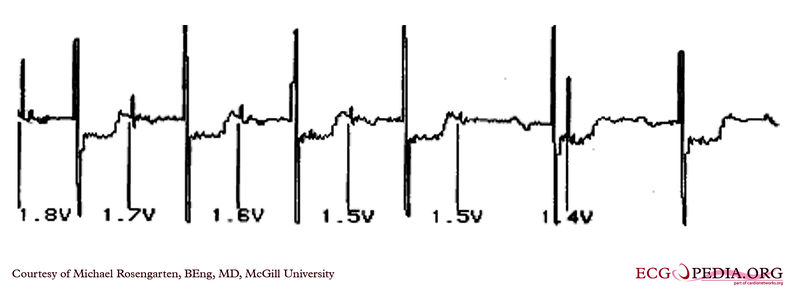
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
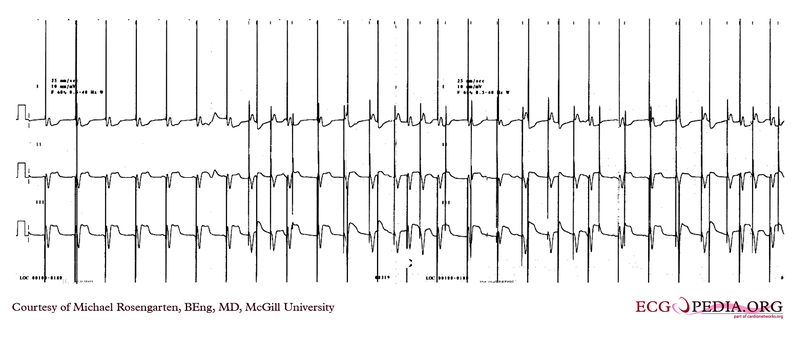
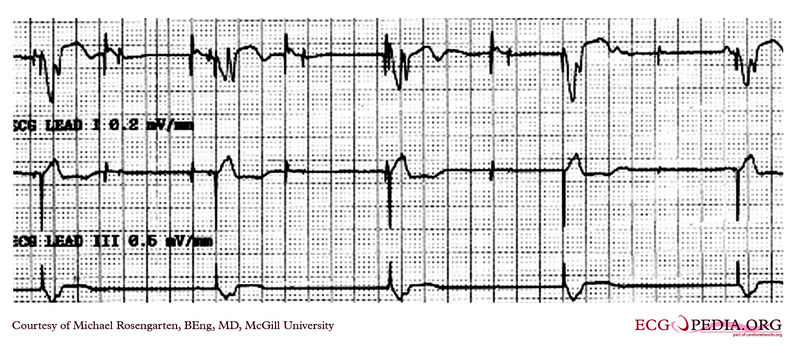
Shown below is an EKG demonstrating threshold test of a VVI pacemaker. Note the effect of the PVCs, the first of which competes with the pacer stimulus and suggests capture when there may be none. Also note that the second to last 0.6 volt pulse captures the ventricle but the last, with the same pulse width, does not.
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
EKG Examples
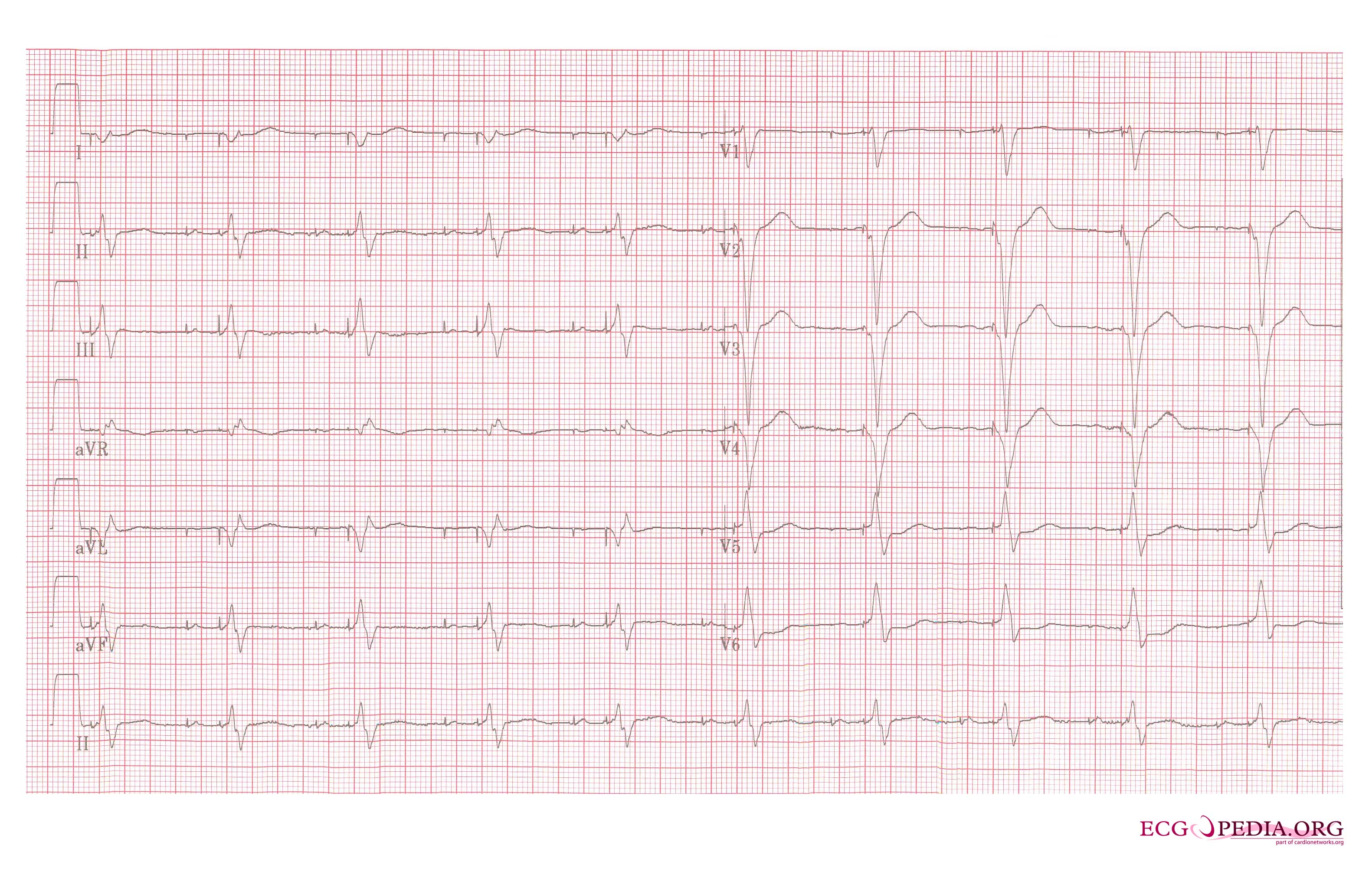
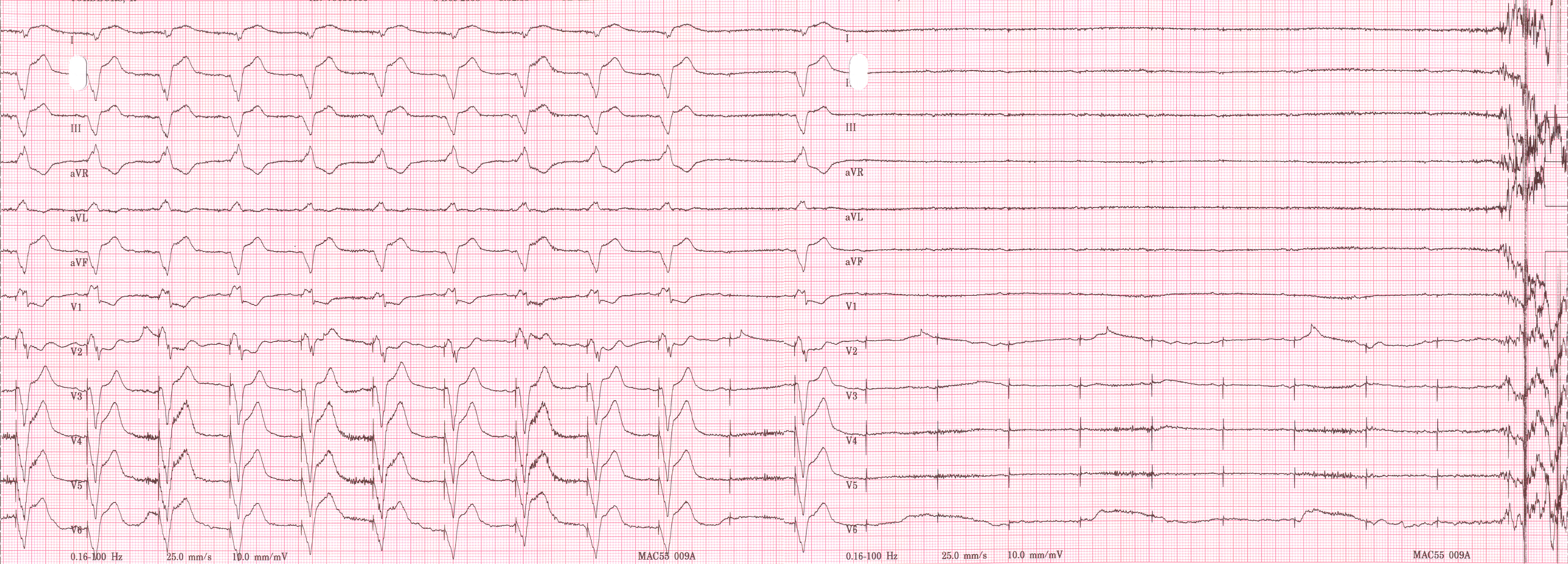
Shown below is an EKG recorded from a patient in his 80's after implantation of a pacemaker. This recording was made as part of a routine pacemaker follow-up The pacing system was working well . This is an interesting electrocardiogram which illustrates an important point regarding pacemaker lead implantation. It is clear that this patient's pacemaker is sensing his P waves and following with the pacing of his ventricle. What is curious about this recording, is the right bundle branch block configuration of the QRS and the extreme rightward axis seen in lead one. It is for this reason, a routine electrocardiogram] should be done after every pacemaker implantation that involves implanting a ventricular lead. In actual fact this patient's ventricular lead was pacing the left ventricle. This lead was probably in a branch of the coronary vein. The path of the lead can be traced by the CT video which was taken after this electrocardiogram was recorded. The patient had a pacemaker system that was working well, in spite of the fact that the pacemaker was pacing the left ventricle. CRT is now available. In this case though, the ventricular lead was removed, without complication, because of an associated infection of the pacemaker pocket. A new pacemaker was implanted from the right side with the new lead placed in the right of the ventricle. This resulted in a markedly different electrocardiogram.

Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
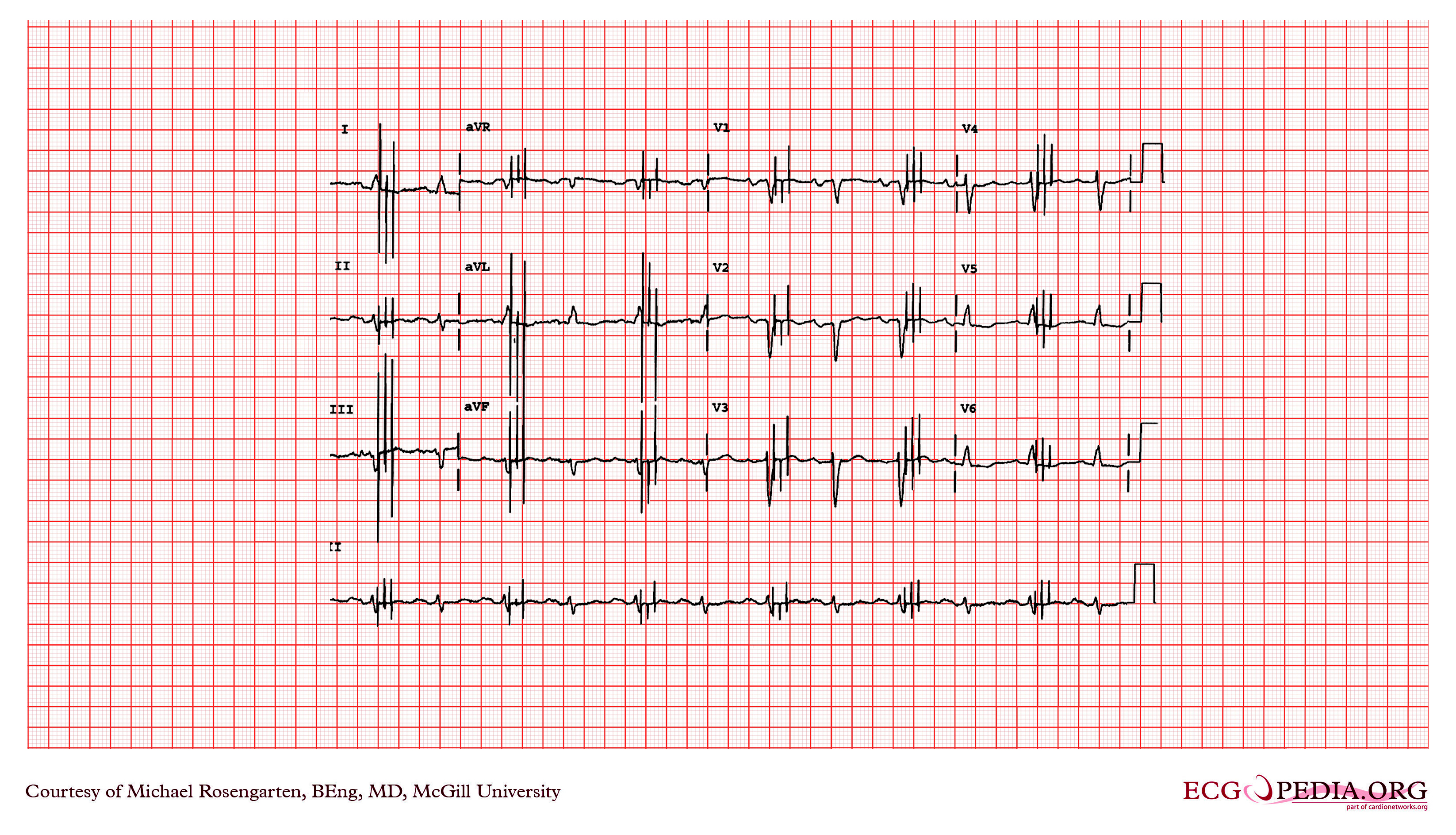
Shown below is an EKG of a 21-year-old patient during threshold testing in the pacemaker clinic. The patient had a congenital heart block for which a pacemaker was implanted. She was asymptomatic before the implant and a dizzy period during the test below.

Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG showing a tracing from the pacemaker clinic with the pacer set slightly below the threshold for capture. A magnet is over the pacemaker.
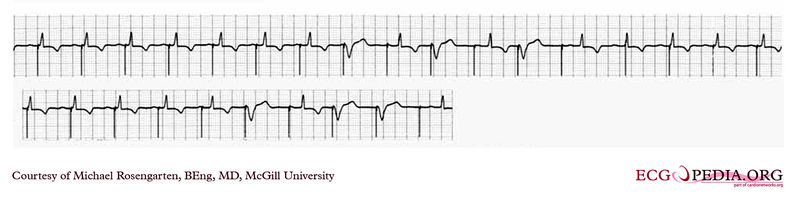
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG showing a tracing from the pacemaker clinic with a Medtronic model 8081 pacer set at 0.18ms @ 0.8 volts slightly below the threshold for capture. A magnet is over the pacemaker.
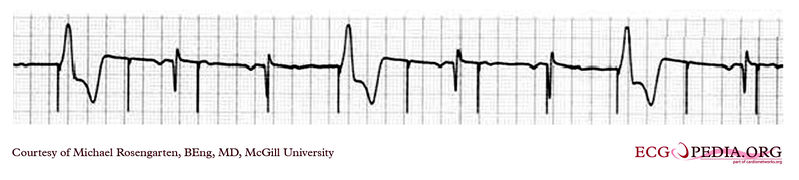
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG showing a tracing from the pacemaker clinic of a pacemaker set at 0.1ms slightly below the threshold for 100% capture. A magnet is over the pacemaker.
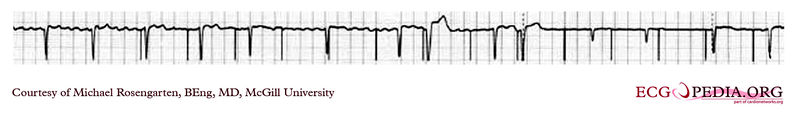
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG example showing time-dependent capture. The native QRS complexes are small (and grow larger due to the auto gain control on this recorder), and the rhythm is probably a nodal escape with sinus arrest. The pacer is a dual-chamber where the atrial lead captures but the ventricular lead captures only if it falls close to a T wave of one of the native escape beats.

Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG example showing tracing in dual-chamber pacemaker in an elderly man.
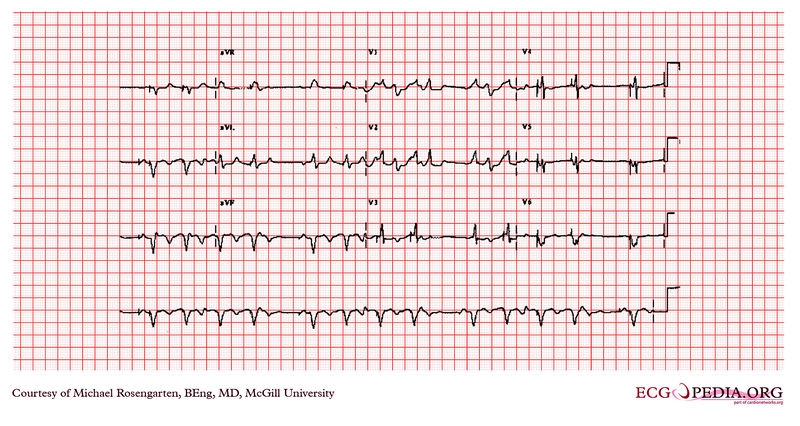
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is the rhythm strip recorded from a man attending the pacemaker follow-up clinic. The patient had been implanted with a ventricular pacemaker one week before. In the middle of the tracing, a magnet was placed over the pacemaker.
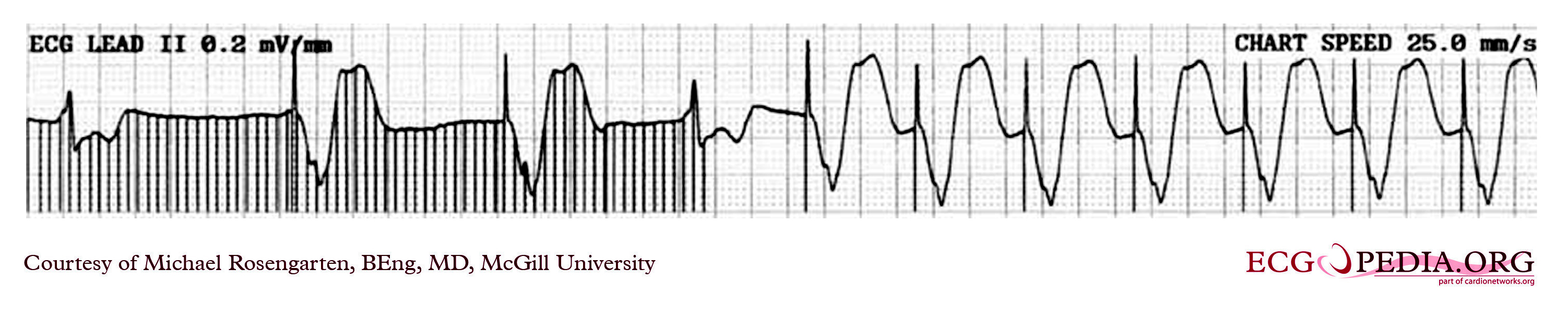
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG tracing taken from an 80-year-old man with a Medtronic Model 8081 unipolar ventricular pacemaker. The patient had come to the clinic for a syncopal episode. During this recording, the patient was doing an isometric exercise with his left arm.
The monitor, in this case, a Medtronic programmer, adds markers to the EKG where it detects pacer spikes. These markers look like pacemaker spikes. Spike detection is controlled by the artifact setting in the programmer, and in some cases, such as in this case, over-detect ( as in the second spike seen above). The pacemaker was set to 60/min. and clearly there is a long period of inhibition of the pacemaker by the pectoral muscle with isometric exercise. This may have been related to the patient's symptoms. The problem was corrected by decreasing the sensitivity of the pacemaker.
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG showing a recording from an elderly woman with complete heart block. She is having an Intermedics Quantum pacemaker (VVI) replaced with a Medtronic Preva DDD pacemaker. The Quantum is implanted in the left pectoral area and is set to V00 mode at 72/min, the Preva is implanted in the right pectoral area and set to VVI at 40/min. A magnet is placed over the Preva near the middle of the tracing. Both pacemakers are in a unipolar mode.
This is an interesting tracing as the two pacemaker leads wherein a similar position in the left ventricle. This is clearly seen as the QRS morphology for both types of paced beats are identical. The morphology is a left bundle like pattern consistent with implantation of the electrode in the right ventricle as desired. Of interest is the difference in the polarity in lead I of the pacer spikes. The spike is positive for the pacer in the left pectoral region and negative for the pacer in the right pectoral area. As the pacemaker is the positive pole and the tip of the electrode the negative pole, this makes sense as lead I have a positive pole on the left shoulder and a negative one on the right.
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG example of a ventricular paced rhythm, with visible ventricular pacemaker spikes.
Shown below is an EKG example showing a patient with a DDD type pace rhythm.
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG demonstrating a patient with a VVI pacemaker rhythm. Note the LBBB morphology with left axis deviation indicating the pacing lead in the right ventricular apex.
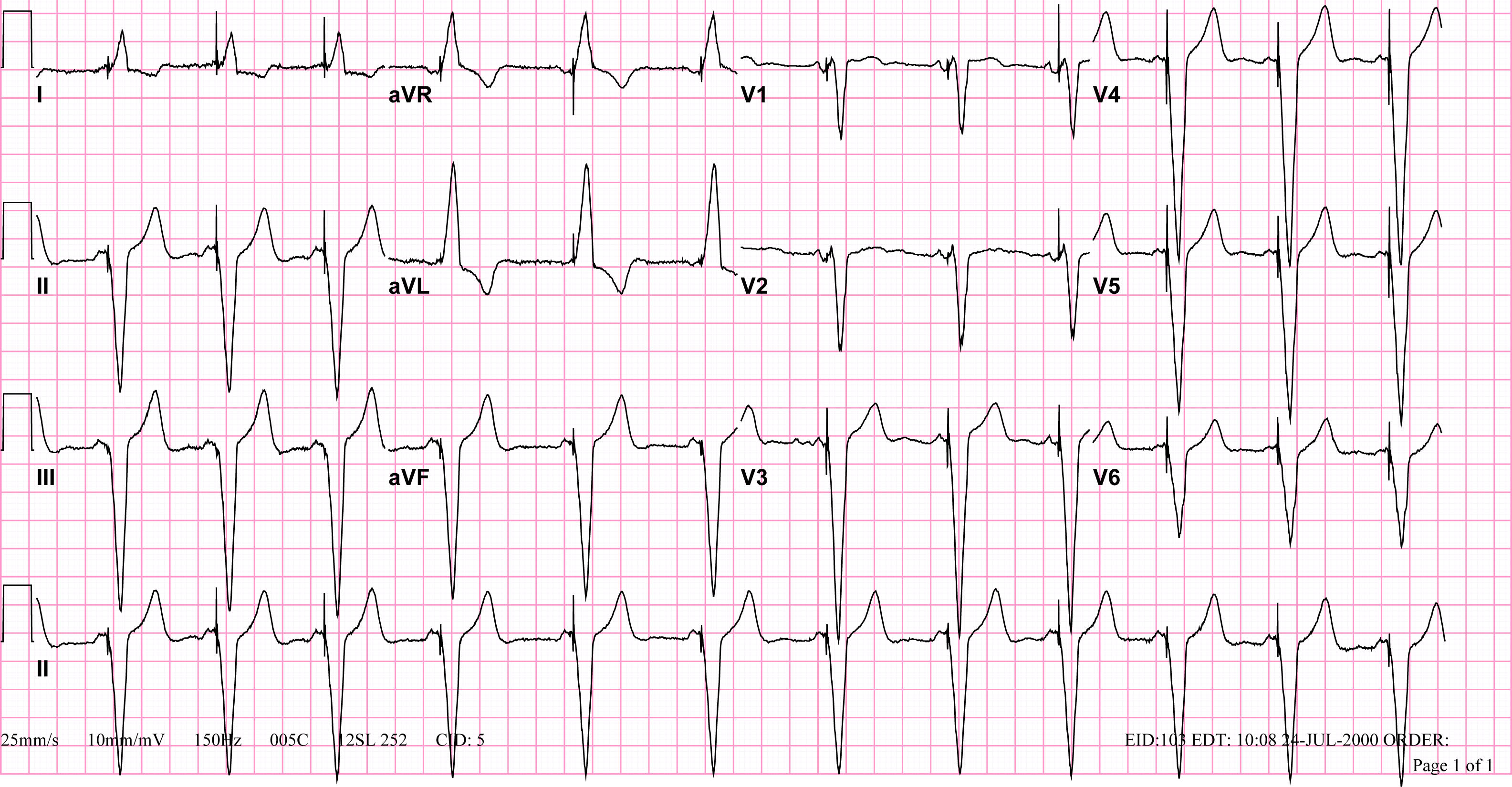
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG showing a ventricular paced rhythm.
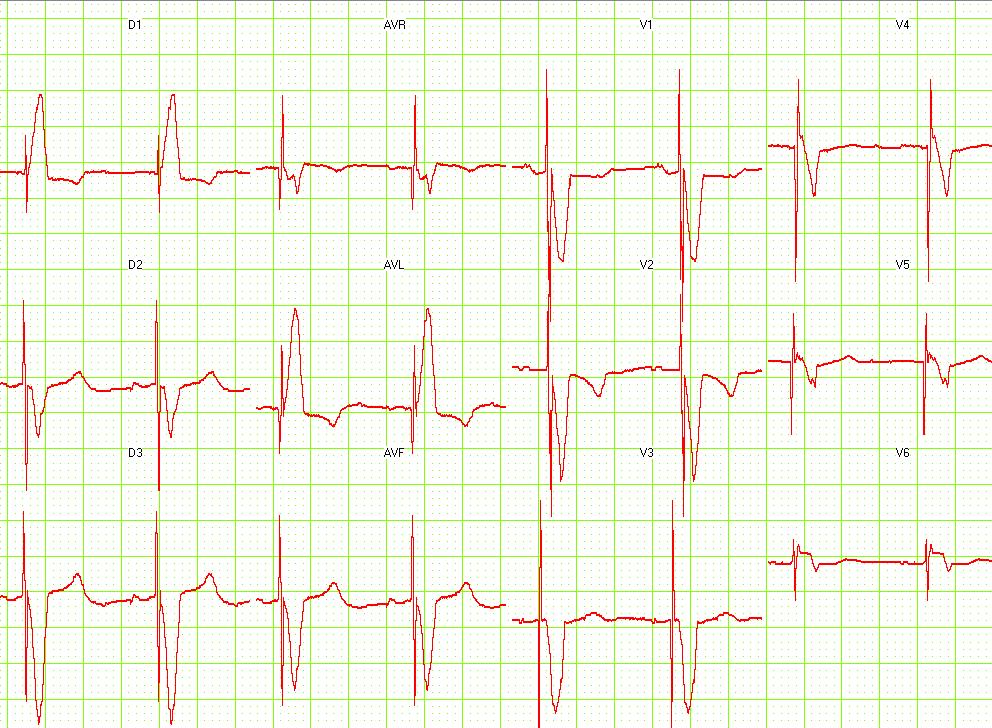
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
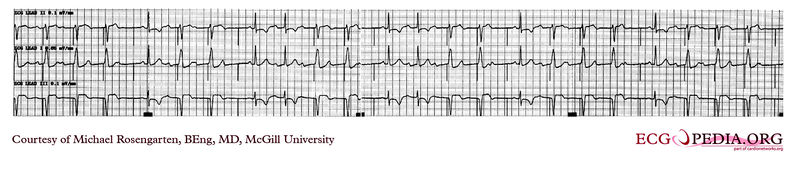
Shown below 12 lead EKG in a person with a dual-chamber pacemaker.
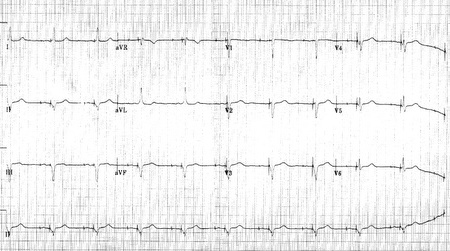
Shown below is an EKG from a patient with a Medtronic model 8081 Premier single chamber pacemaker. The patient is a 65-year-old man who had a history of blackouts while sitting. During the episodes, his wife described him as becoming limp and white. His electrocardiogram showed a left bundle branch block with a normal PR interval. This conduction abnormality had been present for at least 16 years. An electrophysiology test was performed and a pacemaker inserted.
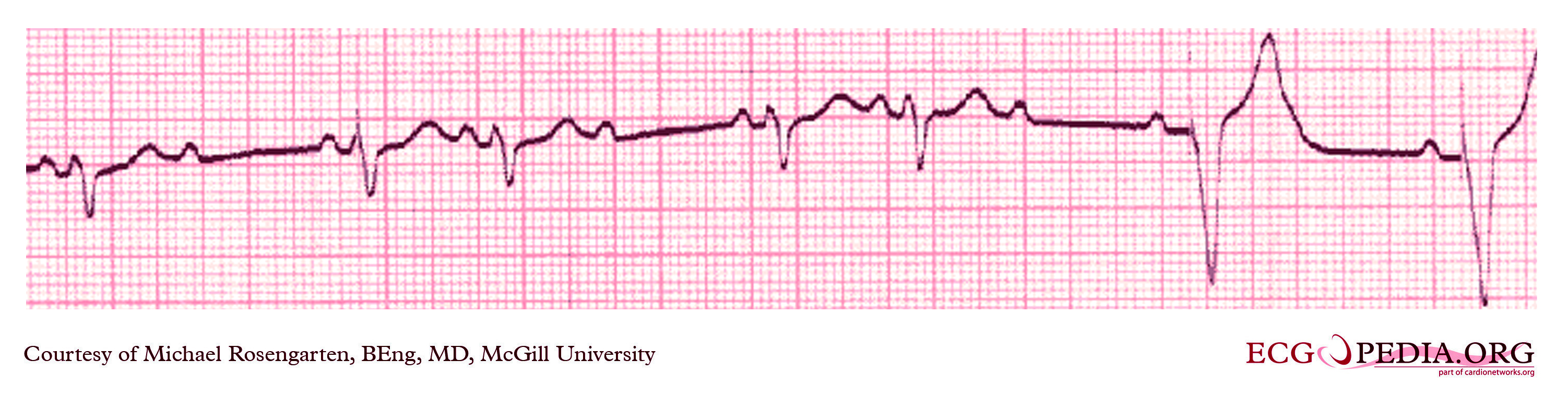
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG showing a ventricular rate of about 72 beats/minute and it would appear that there is a p wave in front of each QRS complex. The "PR" interval is a little short at about 120 ms. The pacer should have started to pace the atrium when set at 100/min. The failure to do so suggests that the pacing system is non-functional (depleted pacemaker battery, broken lead, etc.) or that the pacer is sensing an atrial rhythm faster than 100/min. In this case, the pacer is sensing an SVT with an atrial rate of about 150/min. which explains the lack of pacing when the pacer is set to 100/min. The treating physician decided to leave the patient in this rhythm. It is interesting to note that some SVTs and atrial fibrillation can "cure" a sick sinus patient of their bradycardia and the need for a pacemaker. In those cases, though that convert to atrial fibrillation anticoagulants are usually considered.
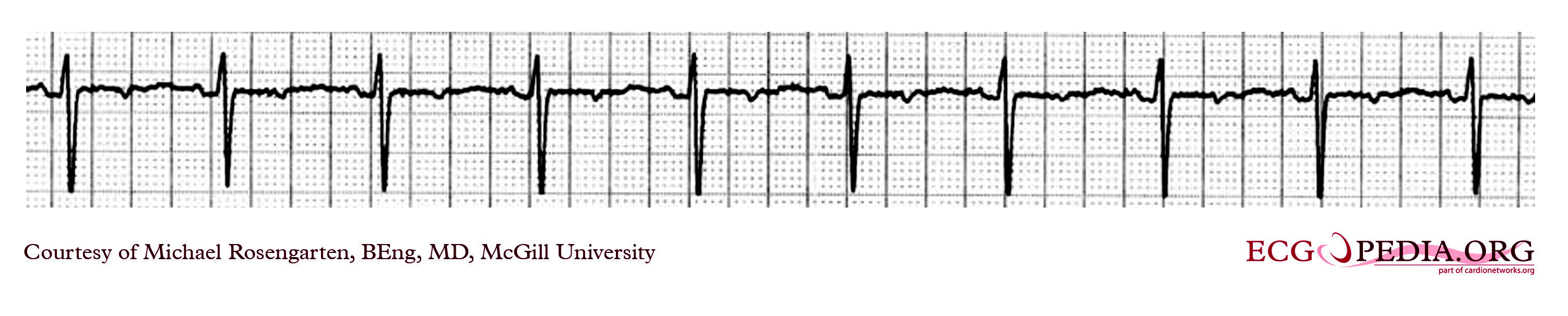
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG example of myopotential inhibition of a VDD pacemaker in unipolar mode. This is a classic recording of myopotential inhibition of a pacemaker. Note the pacemaker that was following the patient's p waves for the first three beats, stops pacing as soon as the baseline records the "buzz" of the pectoral muscle activity. As the pacer stops pacing, the patient's underlying rhythm is seen which appears to be 2:1 AV block.
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below are two tracings from a patient with a history of torsade de pointes and complete heart block. These tracings are from a 24 hour Holter recording taken the day after a pacemaker clinic visit. The visit was prompted by a near syncopal episode. The pacing threshold measured in the clinic was 0.15ms @ 1.6 volts and the patient was discharged with a setting of 0.46ms @ 1.6 volts.

Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG example demonstrating the pacing threshold in a bipolar mode. The lead resistance was 1,140 ohms.
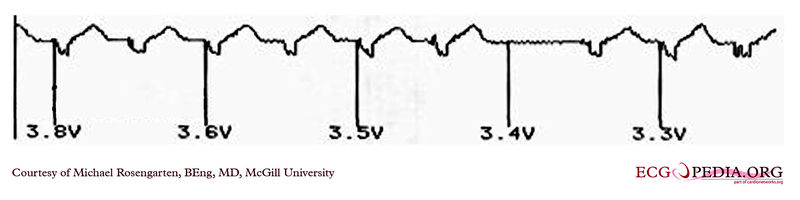
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG demonstrating the pacing threshold in a unipolar mode.
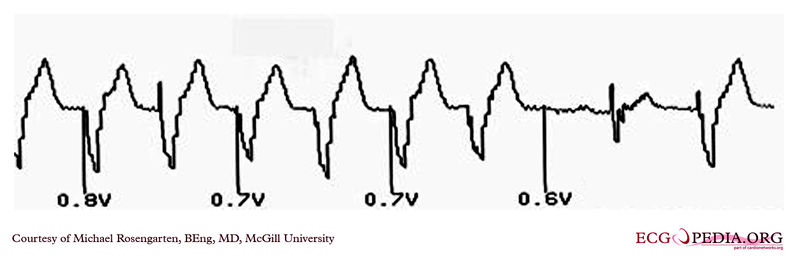
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG example where a magnet was placed over the pacemaker.
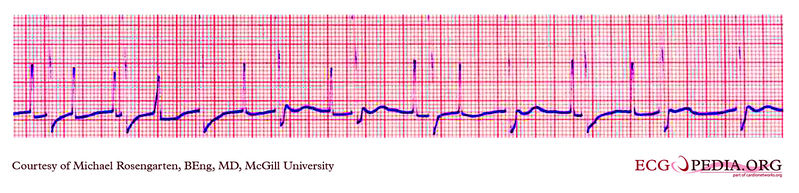
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG of a part of a trans-telephonic strip in a patient with a pacemaker. This section was recorded with a magnet applied over the pacemaker which is a VVI pacemaker set at 50/min.
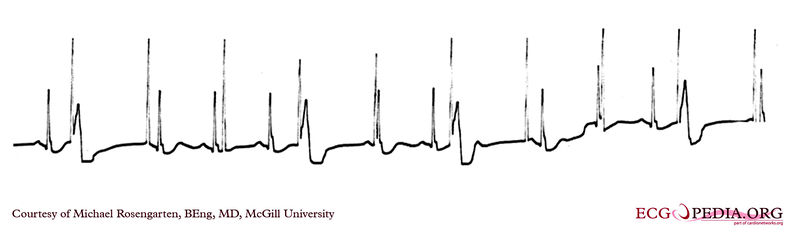
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG strip recording from a patient checking his Medtronic VVI pacemaker. Part of the printout of the rhythm strip is below. Note the 4 fast beats at 100/min near the end of the recording indicating that the patient has placed the magnet properly over the pacemaker.
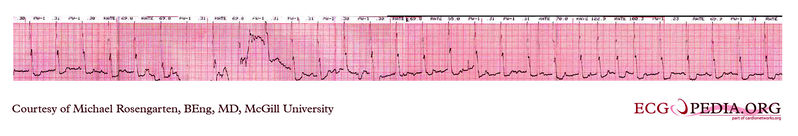
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG tracing from the pacemaker clinic. The patient has a VVI Medtronic pacemaker which was probably set at 70/min. A magnet is placed over the pacemaker and results in a magnet response of 4 beats at 90/min, and then a paced rhythm at about 63/min.
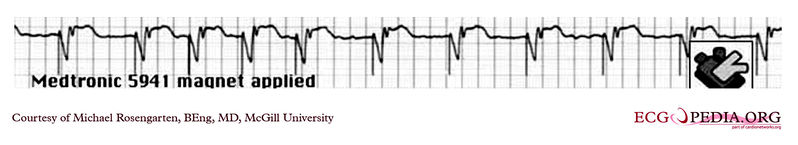
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG strip recording from a patient with a pacemaker. The reason why the pacer was pacing at 75/min in the DDD mode was that there is supraventricular tachycardia (note atrial activity, marked with the turquoise arrows, moving through the ventricular complexes) at about 150/min and the DDD pacer was producing 2:1 A/V block. In the tracing above with the pacemaker set to a VVI mode, the pacemaker no longer senses the atrium and paces at the set rate of 70/min.
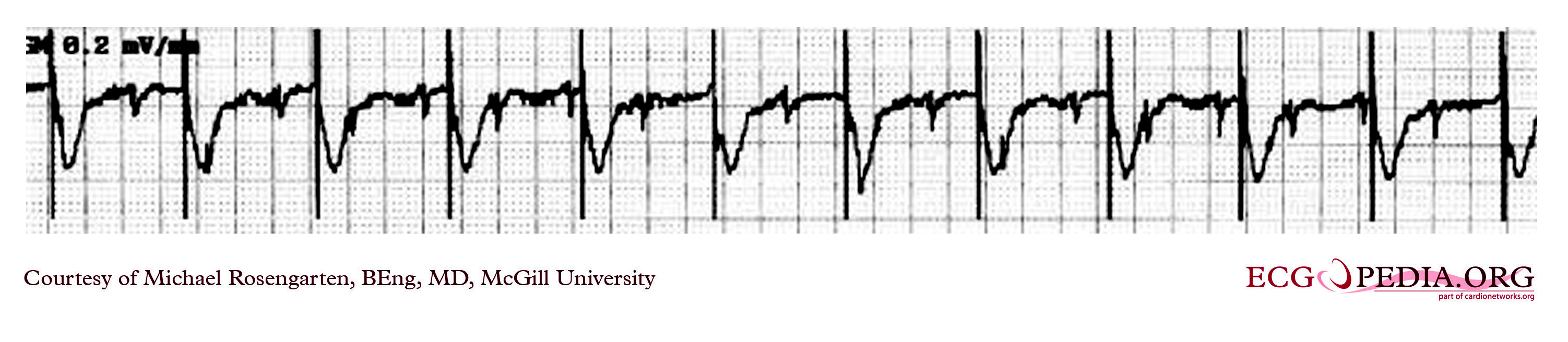
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
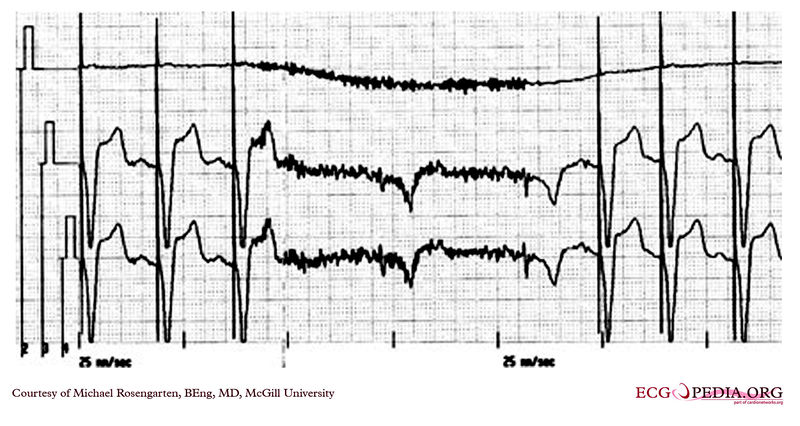
Shown below is an EKG from a patient with a Medtronic model 7108 Minuet dual-chamber pacemaker. The patient had intact A/V conduction, and with a long paced A/V interval, conducted paced atrial beats to the ventricle. The atrial pacing threshold was determined by pacing at a rate faster than the patient's own rate and decreasing the atrial pulse width until atrial capture was lost, and ventricular pacing occurred (the upper tracing).In spite of returning the atrial pulse width back to the last successful value, ventricular pacing continued. Slowing the pacing rate terminated ventricular pacing (the lower tracing) and ventricular pacing did not return when the pacing rate was speeded up again.
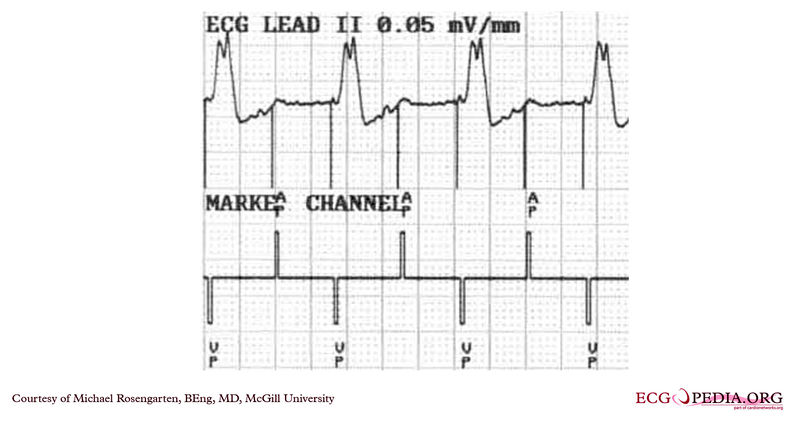
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG from a patient with a Medtronic model 8081 Premier single chamber pacemaker. A patient is a 65-year-old man who had a history of blackouts while sitting. During the episodes, his wife described him as becoming limp and white. His electrocardiogram showed a left bundle branch block with a normal PR interval. This conduction abnormality had been present for at least 16 years. An electrophysiology test was performed and a pacemaker inserted.
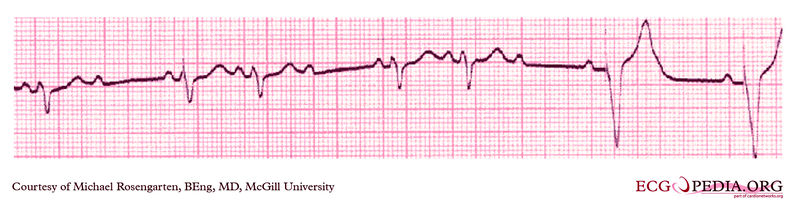
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG rhythm strip recorded from a man attending the pacemaker follow-up clinic. The Patient had been implanted with a ventricular pacemaker one week before. In about the middle of the tracing, a magnet was placed over the pacemaker.
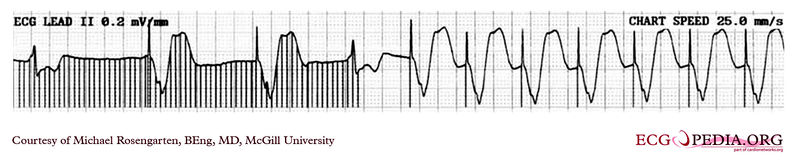
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG rhythm strip recorded from a man attending the pacemaker follow-up clinic. The patient had been implanted with a dual chamber pacemaker one month before. The pacer was programed to VVI mode and AAI mode and ECG recordings were made. Also, a PA and lateral chest x-ray were taken.
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
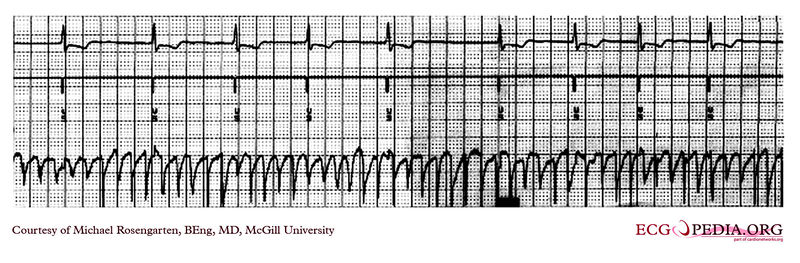
Shown below is an EKG rhythm strip of a threshold determination in a patient with a temporary (epicardial) ventricular pacemaker. The epicardial pacemaker leads were placed after the patient collapsed during aortic valve surgery. In the first half of the tracing, pacemaker stimuli at 60 beats per minute result in a wide QRS complex with a right bundle branch block pattern. Progressively weaker pacing stimuli are administered, which results in asystole in the second half of the tracing. At the end of the tracing, distortion results from muscle contractions due to a (short) hypoxic seizure. Because decreased pacemaker stimuli do not result in a ventricular escape rhythm, the patient can be said to be pacemaker-dependent and needs a definitive pacemaker.
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
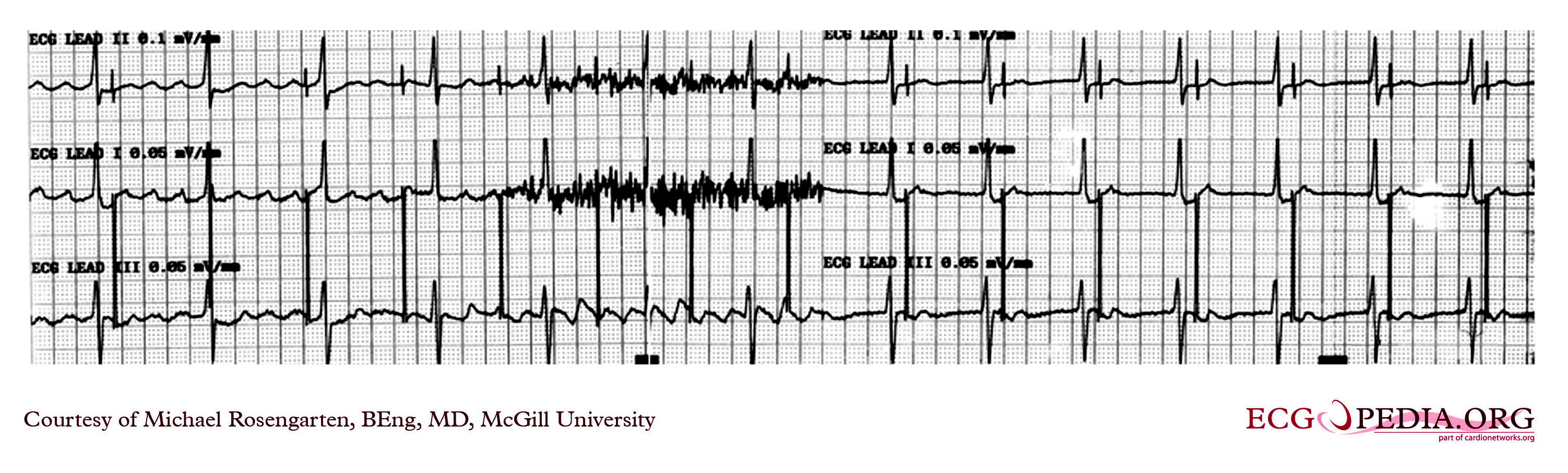
Shown below is an EKG recording of two permanent pacemakers working at the same time. The ventricular pacemaker at a rate of 40/min., while the dual-chamber pacer is set at a rate of about 80/min with an AV interval of about 160 ms. The atrial lead of the dual-chamber pacemaker is capturing and sensing the atrium. The ventricular lead is not capturing the ventricle. There is no native rhythm from the patient seen here.
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG depicting the potential spikes due to a pacemaker. The extra spikes that you see are from a myoplasty stimulator. This is a stimulator used to stimulate a muscle wrapped around the heart. It is stimulating the muscle every second beat. A train is used as this is a skeletal muscle and not a heart muscle.
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG showing the ladder diagram and surface tracing from a patient with a dual chamber pacemaker. The pacer is pacing the atrium (star) and sensing the ventricle. Note the refractory periods (boxes) of the atrium and the ventricle and the post ventricular atrial refractory period.
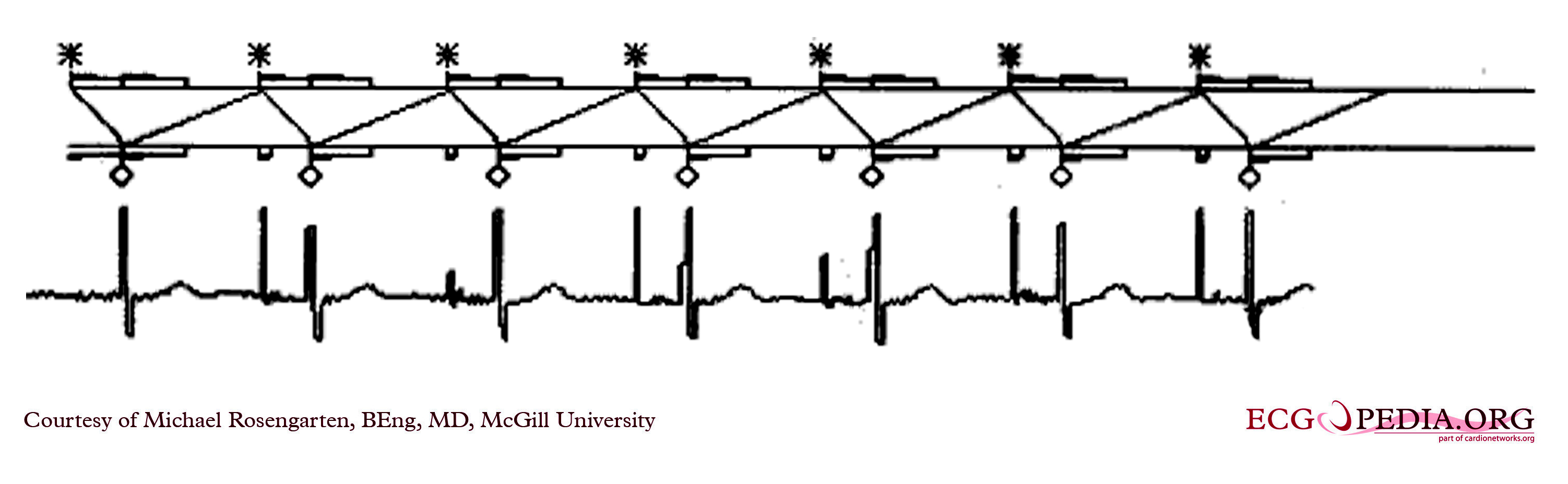
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG demonstrating time-dependent capture. Note the venticular pacemaker captures for several beats but then fails to capture. Capture is restored when the pacer spike falls near to a conducted sinus beat. The underlying rhythm shows a lengthening of the PR interval before a P wave fails to conduct. This suggests a Mobitz I A/V block
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG recording from the atrial lead of a dual chamber pacemaker where the patient is in atrial fibrillation. Note the substantial and rapid atrial signal.
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG recording from a patient coming for a routine DDD pacemaker check.
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an EKG with a magnet placed over the pacemaker that was set at 30/min. Hence it is pacing at 30/min and is not affected by the native sinus beats. This is an interesting case of group beating. The sinus beats are locked into the step with the ventricular pacemaker and suggests an effect of the ventricular paced complex on the sinus node, perhaps as seen with the ventriculophasic effect.
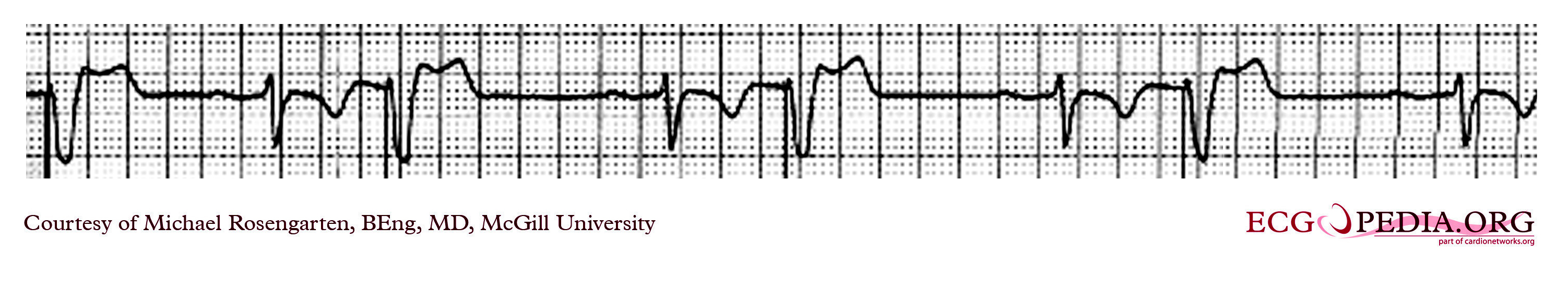
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
Shown below is an intra-operative EKG recording taken during testing of an internal defibrillation system.
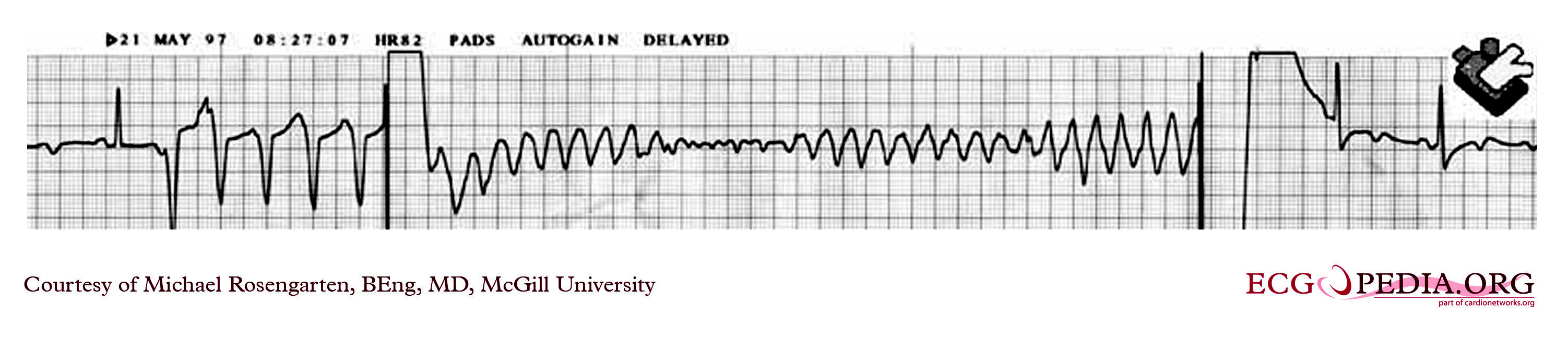
Copyleft image obtained courtesy of ECGpedia, http://en.ecgpedia.org
2012 ACCF/AHA/HRS Focused Updates Incorporated With 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities (DO NOT EDIT)[87][88]
Permanent Pacing in Sinus Node Dysfunction[88]
Acquired Atrioventricular Block in Adults[88]
Permanent Pacing in Chronic Bifascicular Block[88]
Permanent Pacing After the Acute Phase of Myocardial Infarction[88]
Permanent Pacing in Hypersensitive Carotid Sinus Syndrome and Neurocardiogenic Syncope[88]
Pacing After Cardiac Transplantation[88]
Permanent Pacemakers That Automatically Detect and Pace to Terminate Tachycardias[88]
Pacing to Prevent Tachycardia[88]
CRT in Patients With Systolic Heart Failure[87]
Pacing in Patients With Hypertrophic Cardiomyopathy[88]
Permanent Pacing in Children, Adolescents, and Patients With Congenital Heart Disease[88]
Implantable Cardioverter-Defibrillators[88]
Implantable Cardioverter-Defibrillators in Pediatric Patients and Patients With Congenital Heart Disease[88]
Related Chapters
References
- ↑ Rosen, Michael R.; Brink, Peter R.; Cohen, Ira S.; Robinson, Richard B. (2008). "Cardiac Pacing". Circulation: Arrhythmia and Electrophysiology. 1 (1): 54–61. doi:10.1161/CIRCEP.108.764621. ISSN 1941-3149.
- ↑ 2.0 2.1 Chaikhouni A (June 2010). "The magnificent century of cardiothoracic surgery part 8: reviving the dead". Heart Views. 11 (2): 85–91. doi:10.4103/1995-705X.73228. PMC 3000920. PMID 21188005.
- ↑ McWilliam JA (February 1889). "Electrical Stimulation of the Heart in Man". Br Med J. 1 (1468): 348–50. doi:10.1136/bmj.1.1468.348. PMC 2154721. PMID 20752595.
- ↑ Lidwell M C, "Cardiac Disease in Relation to Anaesthesia" in Transactions of the Third Session, Australasian Medical Congress, Sydney, Australia, Sept. 2-7 1929, p 160.
- ↑ 5.0 5.1 Mond H, Sloman J, Edwards R (1982). "The first pacemaker". Pacing and clinical electrophysiology : PACE. 5 (2): 278–82. PMID 6176970.
- ↑ Aquilina O, "A brief history of cardiac pacing", Images Paediatr Cardiol 27 (2006), pp.17-81.
- ↑ Furman S, Szarka G, Layvand D, "Reconstruction of Hyman's second pacemaker", Pacing Clin Electrophysiol.2005 May;28(5):446-453
- ↑ http://www.hno.harvard.edu/gazette/2001/04.19/12-zoll.html
- ↑ Weirich W, Gott V, Lillehei C: The treatment of complete heart block by the combined use of a myocardial electrode and an artificial pacemaker. Surg. Forum 1957;8;360-363
- ↑ "Furman S, Schwedel JB" An intracardiac pacemaker for Stokes-Adams Seizures N Eng J Med 1959; 261:943-948"
- ↑ "Permanent Transvenous Pacing in 1962", Parsonnet V, PACE,1:285, 1978
- ↑ "Preliminary Investigation of the Development of a Permanent Implantable Pacemaker Using an Intracardiac Dipolar Electrode", Parsonnet V, Zucker I R, Asa M M, Clin. Res., 10:391, 1962
- ↑ "How It Happened: My Recollection of Early Pacing", Lageren H, PACE: Pacing and Clinical Electrophysiology 1.1, Jan. 1978, pp 140-143
- ↑ "Intracardiac Stimulation for Complete Heart Block", Lageren H, Acta. Chir. Sca., 125:562, 1963
- ↑ Jean Jaques Welti:Biography, Heart Rhythm Foundation
- ↑ "Medtronic's Minimally Invasive Pacemaker the Size of a Multivitamin". Singularity Hub. 2013-12-27. Retrieved 2013-12-29.
- ↑ "European Post-Approval Trial for Nanostim". DAIC. 2014-03-18.[permanent dead link]
- ↑ "Leadless Pacing from St. Jude Medical". Archived from the original on 2014-10-29. Unknown parameter
|url-status=ignored (help) - ↑ Bereuter L, Gysin M, Kueffer T, Kucera M, Niederhauser T, Fuhrer J, Heinisch P, Zurbuchen A, Obrist D, Tanner H, Haeberlin A (December 2018). "Leadless Dual-Chamber Pacing: A Novel Communication Method for Wireless Pacemaker Synchronization". JACC Basic Transl Sci. 3 (6): 813–823. doi:10.1016/j.jacbts.2018.07.009. PMC 6314974. PMID 30623141.
- ↑ Crawford, TC; Eagle, KA (2017). "Reuse of cardiac implantable electronic devices to improve and extend lives: a call to action". Heart Asia. 9 (1): 34–35. doi:10.1136/heartasia-2016-010835. PMC 5278341. PMID 28191825.
- ↑ 21.0 21.1 Greenspon, Arnold J.; Patel, Jasmine D.; Lau, Edmund; Ochoa, Jorge A.; Frisch, Daniel R.; Ho, Reginald T.; Pavri, Behzad B.; Kurtz, Steven M. (2012). "Trends in Permanent Pacemaker Implantation in the United States From 1993 to 2009". Journal of the American College of Cardiology. 60 (16): 1540–1545. doi:10.1016/j.jacc.2012.07.017. ISSN 0735-1097.
- ↑ . doi:10.4103/1119-3077.224797. Missing or empty
|title=(help) - ↑ Guha A, Xiang X, Haddad D, Buck B, Gao X, Dunleavy M, Liu E, Patel D, Fedorov VV, Daoud EG (August 2017). "Eleven-year trends of inpatient pacemaker implantation in patients diagnosed with sick sinus syndrome". J. Cardiovasc. Electrophysiol. 28 (8): 933–943. doi:10.1111/jce.13248. PMC 5773286. PMID 28471545.
- ↑ 24.0 24.1 Kusumoto, Fred M.; Schoenfeld, Mark H.; Barrett, Coletta; Edgerton, James R.; Ellenbogen, Kenneth A.; Gold, Michael R.; Goldschlager, Nora F.; Hamilton, Robert M.; Joglar, José A.; Kim, Robert J.; Lee, Richard; Marine, Joseph E.; McLeod, Christopher J.; Oken, Keith R.; Patton, Kristen K.; Pellegrini, Cara N.; Selzman, Kimberly A.; Thompson, Annemarie; Varosy, Paul D. (2019). "2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay". Heart Rhythm. 16 (9): e128–e226. doi:10.1016/j.hrthm.2018.10.037. ISSN 1547-5271.
- ↑ O'Gara, Patrick T.; Kushner, Frederick G.; Ascheim, Deborah D.; Casey, Donald E.; Chung, Mina K.; de Lemos, James A.; Ettinger, Steven M.; Fang, James C.; Fesmire, Francis M.; Franklin, Barry A.; Granger, Christopher B.; Krumholz, Harlan M.; Linderbaum, Jane A.; Morrow, David A.; Newby, L. Kristin; Ornato, Joseph P.; Ou, Narith; Radford, Martha J.; Tamis-Holland, Jacqueline E.; Tommaso, Carl L.; Tracy, Cynthia M.; Woo, Y. Joseph; Zhao, David X. (2013). "2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction". Journal of the American College of Cardiology. 61 (4): e78–e140. doi:10.1016/j.jacc.2012.11.019. ISSN 0735-1097.
- ↑ Brignole M, Menozzi C, Moya A, Andresen D, Blanc JJ, Krahn AD, Wieling W, Beiras X, Deharo JC, Russo V, Tomaino M, Sutton R (May 2012). "Pacemaker therapy in patients with neurally mediated syncope and documented asystole: Third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial". Circulation. 125 (21): 2566–71. doi:10.1161/CIRCULATIONAHA.111.082313. PMID 22565936.
- ↑ da Silva RM (2016). "The Current Indication for Pacemaker in Patients with Cardioinhibitory Vasovagal Syncope". Open Cardiovasc Med J. 10: 179–87. doi:10.2174/1874192401610010179. PMC 5009292. PMID 27651841.
- ↑
Barón-Esquivias G, Barón-Solís C, Ordóñez A (2019). "Pacing for Patients Suffering From Cardioinhibitory Vasovagal Syncope Using the Closed-Loop System". Front Cardiovasc Med. 6: 192. doi:10.3389/fcvm.2019.00192. PMC 7033422 Check
|pmc=value (help). PMID 32118042 Check|pmid=value (help). - ↑ Gopinathannair R, Salgado BC, Olshansky B (June 2018). "Pacing for Vasovagal Syncope". Arrhythm Electrophysiol Rev. 7 (2): 95–102. doi:10.15420/aer.2018.22.2. PMC 6020179. PMID 29967681.
- ↑ Villain, Elisabeth (2008). "Indications for Pacing in Patients with Congenital Heart Disease". Pacing and Clinical Electrophysiology. 31: S17–S20. doi:10.1111/j.1540-8159.2008.00948.x. ISSN 0147-8389.
- ↑ Khazanie P, Hellkamp AS, Fonarow GC, Curtis LH, Al-Khatib SM, Hernandez AF (April 2018). "Permanent pacemaker use among patients with heart failure and preserved ejection fraction: Findings from the Acute Decompensated Heart Failure National Registry (ADHERE) National Registry". Am. Heart J. 198: 123–128. doi:10.1016/j.ahj.2017.12.020. PMID 29653633.
- ↑ "2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy". European Heart Journal. 35 (39): 2733–2779. 2014. doi:10.1093/eurheartj/ehu284. ISSN 0195-668X.
- ↑ Tolosana, José Maria; Trucco, Emilce (2018). "Cardiac pacing in patients with hypertrophic obstructive cardiomyopathy". Global Cardiology Science and Practice. 2018 (3). doi:10.21542/gcsp.2018.29. ISSN 2305-7823.
- ↑ Zareba, Wojciech; Moss, Arthur J.; Daubert, James P.; Hall, W. Jackson; Robinson, Jennifer L.; Andrews, Mark (2003). "Implantable Cardioverter Defibrillator in High‐Risk Long QT Syndrome Patients". Journal of Cardiovascular Electrophysiology. 14 (4): 337–341. doi:10.1046/j.1540-8167.2003.02545.x. ISSN 1045-3873.
- ↑ McCann P (January 2007). "A review of temporary cardiac pacing wires". Indian Pacing Electrophysiol J. 7 (1): 40–9. PMC 1764908. PMID 17235372.
- ↑ Tjong, F. V. Y.; de Ruijter, U. W.; Beurskens, N. E. G.; Knops, R. E. (2019). "A comprehensive scoping review on transvenous temporary pacing therapy". Netherlands Heart Journal. 27 (10): 462–473. doi:10.1007/s12471-019-01307-x. ISSN 1568-5888.
- ↑ Metkus, Thomas S.; Schulman, Steven P.; Marine, Joseph E.; Eid, Shaker M. (2019). "Complications and Outcomes of Temporary Transvenous Pacing". Chest. 155 (4): 749–757. doi:10.1016/j.chest.2018.11.026. ISSN 0012-3692.
- ↑ Doukky R, Bargout R, Kelly RF, Calvin JE (May 2003). "Using transcutaneous cardiac pacing to best advantage: How to ensure successful capture and avoid complications". J Crit Illn. 18 (5): 219–225. PMC 6376978. PMID 30774278.
- ↑ 39.0 39.1 Batra, AnjanS; Balaji, Seshadri (2008). "Post operative temporary epicardial pacing: When, how and why?". Annals of Pediatric Cardiology. 1 (2): 120. doi:10.4103/0974-2069.43877. ISSN 0974-2069.
- ↑ Verbeet T, Castro J, Decoodt P (October 2003). "Transesophageal pacing: a versatile diagnostic and therapeutic tool". Indian Pacing Electrophysiol J. 3 (4): 202–9. PMC 1502053. PMID 16943920.
- ↑ Roth JV (September 1996). "Phrenic nerve stimulation during transesophageal atrial pacing may cause apnea in spontaneously breathing patients". Anesth. Analg. 83 (3): 661. doi:10.1097/00000539-199609000-00054. PMID 8780310.
- ↑ (Cite_Journal)Percussion pacing in a three-year-old girl with complete heart block during cardiac catheterization. C Eich, A Bleckmann and T. Paul, retrieved from http://bja.oxfordjournals.org/cgi/content/full/95/4/465
- ↑ Eich, C.; Bleckmann, A.; Schwarz, S.K.W. (2007). "Percussion pacing—an almost forgotten procedure for haemodynamically unstable bradycardias? A report of three case studies and review of the literature". British Journal of Anaesthesia. 98 (4): 429–433. doi:10.1093/bja/aem007. ISSN 0007-0912.
- ↑ Siddiqui AM, Harris GS, Movahed A, Chiang KS, Chelu MG, Nekkanti R (September 2015). "Transhepatic venous approach to permanent pacemaker placement in a patient with limited central venous access". World J Clin Cases. 3 (9): 835–7. doi:10.12998/wjcc.v3.i9.835. PMC 4568533. PMID 26380831.
- ↑ Bhatia N, El-Chami M (April 2018). "Leadless pacemakers: a contemporary review". J Geriatr Cardiol. 15 (4): 249–253. doi:10.11909/j.issn.1671-5411.2018.04.002. PMC 5997619. PMID 29915613.
- ↑
Wilkoff BL, Cook JR, Epstein AE; et al. (2002). "Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial". JAMA. 288 (24): 3115–23. PMID 12495391. Unknown parameter
|month=ignored (help) - ↑ Zhang Q, Yu CM (September 2012). "Clinical implication of mechanical dyssynchrony in heart failure". J Cardiovasc Ultrasound. 20 (3): 117–23. doi:10.4250/jcu.2012.20.3.117. PMC 3498307. PMID 23185653.
- ↑ Cleland JG, Daubert JC, Erdmann E; et al. (2005). "The effect of cardiac resynchronization on morbidity and mortality in heart failure". N. Engl. J. Med. 352 (15): 1539–49. doi:10.1056/NEJMoa050496. PMID 15753115.
- ↑ Bardy GH, Lee KL, Mark DB; et al. (2005). "Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure". N. Engl. J. Med. 352 (3): 225–37. doi:10.1056/NEJMoa043399. PMID 15659722.
- ↑ Cleland J, Daubert J, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L (2005). "The effect of cardiac resynchronization on morbidity and mortality in heart failure". N Engl J Med. 352 (15): 1539–49. doi:10.1056/NEJMoa050496. PMID 15753115.
- ↑ Ganjehei L, Razavi M, Massumi A (2011). "Cardiac resynchronization therapy: a decade of experience and the dilemma of nonresponders". Tex Heart Inst J. 38 (4): 358–60. PMC 3147217. PMID 21841860.
- ↑ Bristow M, Saxon L, Boehmer J, Krueger S, Kass D, De Marco T, Carson P, DiCarlo L, DeMets D, White B, DeVries D, Feldman A (2004). "Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure". N Engl J Med. 350 (21): 2140–50. doi:10.1056/NEJMoa032423. PMID 15152059.
- ↑ Dell'Orto S, Valli P, Greco EM (July 2004). "Sensors for rate-responsive pacing". Indian Pacing Electrophysiol J. 4 (3): 137–45. PMC 1501080. PMID 16943981.
- ↑ Coenen M, Malinowski K, Spitzer W, Schuchert A, Schmitz D, Anelli-Monti M, Maier SK, Estlinbaum W, Bauer A, Muehling H, Kalscheur F, Puerner K, Boergel J, Osswald S (March 2008). "Closed loop stimulation and accelerometer-based rate adaptation: results of the PROVIDE study". Europace. 10 (3): 327–33. doi:10.1093/europace/eun024. PMID 18272507.
- ↑ Cicchitti, Vincenzo; Radico, Francesco; Bianco, Francesco; Gallina, Sabina; Tonti, Gianni; De Caterina, Raffaele (2016). "Heart failure due to right ventricular apical pacing: the importance of flow patterns". Europace. 18 (11): 1679–1688. doi:10.1093/europace/euw024. ISSN 1099-5129.
- ↑ "Focus on Electrophysiology: His Bundle Pacing: A More Physiologic Alternative For Pacing". American College of Cardiology. April 26, 2019.
- ↑ Sharma PS, Vijayaraman P, Ellenbogen KA (January 2020). "Permanent His bundle pacing: shaping the future of physiological ventricular pacing". Nat Rev Cardiol. 17 (1): 22–36. doi:10.1038/s41569-019-0224-z. PMID 31249403.
- ↑ Abdelrahman, Mohamed; Subzposh, Faiz A.; Beer, Dominik; Durr, Brendan; Naperkowski, Angela; Sun, Haiyan; Oren, Jess W.; Dandamudi, Gopi; Vijayaraman, Pugazhendhi (2018). "Clinical Outcomes of His Bundle Pacing Compared to Right Ventricular Pacing". Journal of the American College of Cardiology. 71 (20): 2319–2330. doi:10.1016/j.jacc.2018.02.048. ISSN 0735-1097.
- ↑ Zanon F, Ellenbogen KA, Dandamudi G, Sharma PS, Huang W, Lustgarten DL, Tung R, Tada H, Koneru JN, Bergemann T, Fagan DH, Hudnall JH, Vijayaraman P (November 2018). "Permanent His-bundle pacing: a systematic literature review and meta-analysis". Europace. 20 (11): 1819–1826. doi:10.1093/europace/euy058. PMID 29701822.
- ↑ Böhm A, Pintér A, Székely A, Préda I (1998). "Clinical Observations with Long-term Atrial Pacing". Pacing Clin Electrophysiol. 21 (1): 246–9. doi:10.1111/j.1540-8159.1998.tb01097.x. PMID 9474681.
- ↑ Crick JC (1991). "European Multicenter Prospective Follow-Up Study of 1,002 Implants of a Single Lead VDD Pacing System". Pacing Clin Electrophysiol. 14 (11): 1742–4. doi:10.1111/j.1540-8159.1991.tb02757.x. PMID 1749727.
- ↑ Bernstein A, Daubert J, Fletcher R, Hayes D, Lüderitz B, Reynolds D, Schoenfeld M, Sutton R (2002). "The revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group". Pacing Clin Electrophysiol. 25 (2): 260–4. PMID 11916002.
- ↑ Bristow, Michael R.; Saxon, Leslie A.; Boehmer, John; Krueger, Steven; Kass, David A.; De Marco, Teresa; Carson, Peter; DiCarlo, Lorenzo; DeMets, David; White, Bill G.; DeVries, Dale W.; Feldman, Arthur M. (2004). "Cardiac-Resynchronization Therapy with or without an Implantable Defibrillator in Advanced Chronic Heart Failure". New England Journal of Medicine. 350 (21): 2140–2150. doi:10.1056/NEJMoa032423. ISSN 0028-4793.
- ↑ 64.0 64.1 Bernstein A, Camm A, Fisher J, Fletcher R, Mead R, Nathan A, Parsonnet V, Rickards A, Smyth N, Sutton R (1993). "North American Society of Pacing and Electrophysiology policy statement. The NASPE/BPEG defibrillator code". Pacing Clin Electrophysiol. 16 (9): 1776–80. PMID 7692407.
- ↑ Basil A, Lubitz SA, Noseworthy PA, Reynolds MR, Gold H, Yassa D, Kramer D (June 2017). "Periprocedural Antibiotic Prophylaxis for Cardiac Implantable Electrical Device Procedures: Results From a Heart Rhythm Society Survey". JACC Clin Electrophysiol. 3 (6): 632–634. doi:10.1016/j.jacep.2017.01.013. PMC 5550102. PMID 28804785.
- ↑ Ramsdale, David R. (2012). Cardiac pacing and device therapy. Rao, Archana. London: Springer. ISBN 978-1-4471-2939-4. OCLC 822576869.
- ↑ European Pacemaker Patient Identification card
- ↑ Eucomed
- ↑ Roberts PR (September 2005). "Follow up and optimisation of cardiac pacing". Heart. 91 (9): 1229–34. doi:10.1136/hrt.2004.054528. PMC 1769065. PMID 16103575.
- ↑ "Testing of work environments for electromagnetic interference (Pacing Clin Electrophysiol. 1992) - PubMed Result". www.ncbi.nlm.nih.gov. Retrieved 2008-11-10.
- ↑ "Potential Cell Phone Interference with Pacemakers and Other Medical Devices | FDA".
- ↑ "MP3 Headphones Interfere With Implantable Defibrillators, Pacemakers - Beth Israel Deaconess Medical Center". www.bidmc.org. Retrieved 2008-11-10.
- ↑ Shea, Julie B.; Aguilar, Martin; Sauer, William H.; Tedrow, Usha (2020). "Unintentional magnet reversion of an implanted cardiac defibrillator by an electronic cigarette". HeartRhythm Case Reports. 6 (3): 121–123. doi:10.1016/j.hrcr.2020.01.013. ISSN 2214-0271.
- ↑ Kolb, Christof; Schmieder, Sebastian; Lehmann, Günter; Zrenner, Bernhard; Karch, Martin R; Plewan, Andreas; Schmitt, Claus (2003). "Do airport metal detectors interfere with implantable pacemakers or cardioverter-defibrillators?". Journal of the American College of Cardiology. 41 (11): 2054–2059. doi:10.1016/S0735-1097(03)00424-8. ISSN 0735-1097.
- ↑ Ubee, Sarvpreet Singh; Kasi, Vijaykumar S.; Bello, David; Manikandan, Ramaswamy (2011). "Implications of Pacemakers and Implantable Cardioverter Defibrillators in Urological Practice". Journal of Urology. 186 (4): 1198–1205. doi:10.1016/j.juro.2011.02.2697. ISSN 0022-5347.
- ↑ Ferreira, António M; Costa, Francisco; Tralhão, António; Marques, Hugo; Cardim, Nuno; Adragão, Pedro (7 May 2014). "MRI-conditional pacemakers: current perspectives". Medical Devices. 7: 115–124. doi:10.2147/MDER.S44063. PMC 4019608. PMID 24851058.
- ↑ Halperin, Daniel (2008). Pacemakers and Implantable Cardiac Defibrillators: Software Radio Attacks and Zero-Power Defenses (PDF). IEEE Symposium on Security and Privacy. Retrieved 2008-08-10. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ "Researchers Develop Personal Firewall Solution for Pacemakers, Insulin Pumps". eSecurityPlanet.com. 2012-04-20. Retrieved 2012-04-20.
- ↑ "Heart devices can be turned off near end of life". amednews.com. May 31, 2010.
- ↑ Kapa S, Mueller PS, Hayes DL, Asirvatham SJ (November 2010). "Perspectives on withdrawing pacemaker and implantable cardioverter-defibrillator therapies at end of life: results of a survey of medical and legal professionals and patients". Mayo Clin. Proc. 85 (11): 981–90. doi:10.4065/mcp.2010.0431. PMC 2966361. PMID 20843982.
- ↑ "Risks - Pacemaker - Mayo Clinic". www.mayoclinic.org. Retrieved 2016-12-01.
- ↑ Hayes DL, Loesl K (July 2002). "Pacemaker component allergy: case report and review of the literature". J Interv Card Electrophysiol. 6 (3): 277–8. doi:10.1023/a:1019518005809. PMID 12154330.
- ↑ 83.0 83.1 Pacemaker-Mediated Tachycardia at eMedicine
- ↑ Transvenous Lead Extraction: Heart Rhythm Society Expert Consensus on Facilities, Training, Indications, and Patient Management Archived 2014-12-12 at the Wayback Machine. Author: Bruce L. Wilkoff, MD. Coauthor(s): Charles J. Love, MD, FHRS, Charles L. Byrd, MD, Maria Grazia Bongiorni, MD, Roger G. Carrillo, MD, FHRS, George H. Crossley, III, MD, FHRS, Laurence M. Epstein, MD, Richard A. Friedman, MD, MBA, FHRS, Charles E. H. Kennergren, MD, PhD, FHRS, Przemyslaw Mitkowski, MD, Raymond H. M. Schaerf, MD, FHRS, Oussama M. Wazni, MD
- ↑ Kalavakunta, Jagadeesh Kumar; Gupta, Vishal; Paulus, Basil; Lapenna, William (2014). "An Unusual Cause of Transient Ischemic Attack in a Patient with Pacemaker". Case Reports in Cardiology. 2014: 265759. doi:10.1155/2014/265759. PMC 4008350. PMID 24826308.
- ↑ Salahuddin M, Cader FA, Nasrin S, Chowdhury MZ (January 2016). "The pacemaker-twiddler's syndrome: an infrequent cause of pacemaker failure". BMC Res Notes. 9: 32. doi:10.1186/s13104-015-1818-0. PMC 4721019. PMID 26790626.
- ↑ 87.0 87.1 Tracy CM, Epstein AE, Darbar D, Dimarco JP, Dunbar SB, Estes NA, Ferguson TB, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Stevenson WG, Varosy PD (2012). "2012 ACCF/AHA/HRS Focused Update of the 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines". Heart Rhythm. 9 (10): 1737–53. doi:10.1016/j.hrthm.2012.08.021. PMID 22975672. Retrieved 2012-11-01. Unknown parameter
|month=ignored (help) - ↑ 88.00 88.01 88.02 88.03 88.04 88.05 88.06 88.07 88.08 88.09 88.10 88.11 88.12 Epstein AE, Dimarco JP, Ellenbogen KA, Estes NA, Freedman RA, Gettes LS; et al. (2008). "ACC/AHA/HRS 2008 guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: executive summary". Heart Rhythm. 5 (6): 934–55. doi:10.1016/j.hrthm.2008.04.015. PMID 18534377.
- Pages with reference errors
- CS1 maint: Multiple names: authors list
- All articles with dead external links
- Articles with dead external links from July 2017
- Articles with invalid date parameter in template
- Articles with permanently dead external links
- Pages with citations using unsupported parameters
- Pages with citations lacking titles
- CS1 errors: PMC
- CS1 errors: PMID
- CS1 maint: Explicit use of et al.
- Webarchive template wayback links
- Electrophysiology
- Cardiology
- Arrhythmia