Varicella zoster virus
For information about primary infection with VZV, visit chicken pox.
For information about reactivation of primary VZV infection, visit shingles.
For information about congenital Varicella Syndrome, click here.
|
Chickenpox Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Varicella zoster virus On the Web |
|
American Roentgen Ray Society Images of Varicella zoster virus |
|
Risk calculators and risk factors for Varicella zoster virus |
| Varicella zoster virus | ||||||||
|---|---|---|---|---|---|---|---|---|
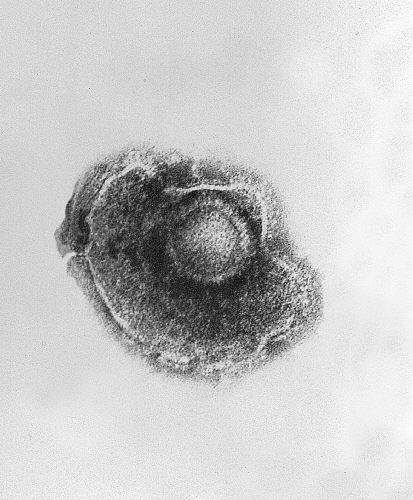 Micrograph of VZV.
| ||||||||
| Virus classification | ||||||||
| ||||||||
| Species | ||||||||
|
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Varicella zoster virus or varicella-zoster virus (VZV) is one of eight herpes viruses known to infect humans and vertebrates. VZV only affects humans, and commonly causes chickenpox in children, teens and young adults and herpes zoster (shingles) in adults and rarely in children. VZV is known by many names, including chickenpox virus, varicella virus, zoster virus, and human herpesvirus type 3 (HHV-3).
VZV infects the nerves and causes a wide variety of symptoms. After the primary infection (chickenpox), the virus goes dormant in the nerves, including the cranial nerve ganglia, dorsal root ganglia, and autonomic ganglia. Many years after the patient has recovered from chickenpox, VZV can reactivate to cause a number of neurologic conditions.[1]
Human disease
Primary Varicella Zoster Virus infection results in chickenpox (varicella), which may result in complications including encephalitis or pneumonia (either direct viral pneumonia or secondary bacterial pneumonia). Even when clinical symptoms of chickenpox have resolved, VZV remains dormant in the nervous system of the infected person (virus latency), in the trigeminal and dorsal root ganglia.[2]
In about 10–20% of cases, VZV reactivates later in life, producing a disease known as shingles or herpes zoster. VZV can also infect the central nervous system, with a 2013 article reporting an incidence rate of 1.02 cases per 100 000 inhabitants in Switzerland, and an annual incidence rate of 1.8 cases per 100,000 inhabitants in Sweden.[3] For comparison, Amyotrophic lateral sclerosis (ALS) has an annual incidence rate of 0.2 to 2.4 per 100,000 population in industrialized countries. [4]
Other serious complications of varicella zoster infection include postherpetic neuralgia, Mollaret's meningitis, zoster multiplex, and inflammation of arteries in the brain leading to stroke,[5] myelitis, herpes ophthalmics, or zoster sine herpete. In Ramsay Hunt syndrome, VZV affects the geniculate ganglion resulting in lesions that follow specific branches of the facial nerve. Symptoms may include painful blisters on the tongue and ear along with one sided facial weakness and hearing loss.
Recent advances in research and diagnosis
Until the mid-1990s, infectious complications of the CNS caused by VZV reactivation were regarded as rare. The presence of rash, as well as specific neurological symptoms, were required to diagnose a CNS infection caused by VZV. Since 2000, PCR testing has become more widely used, and the number of diagnosed cases of CNS infection has increased. [6]
Classic textbook descriptions state that VZV reactivation in the CNS is restricted to immunocompromised individuals and the elderly, however, recent studies have found that most patients are immunocompetent, and less than 60 years old. Old references cite vesicular rash as a characteristic finding, however, recent studies have found that the rash is only present in 45% of cases. [7] In addition, systemic inflammation is not as reliable an indicator as previously thought: the mean level of C-reactive protein and mean white blood cell count is within the normal range in patients with VZV meningitis. [8] MRI and CT scans are usually normal in cases of VZV reactivation in the CNS. CSF pleocytosis, previously thought to be a strong indicator of VZV encephalitis, was absent in half of a group of patients diagnosed with VZV encephalitis by PCR. [9]
The frequency of CNS infections presented at the emergency room of a community hospital is not negligible, so a means of diagnosing cases is needed. PCR is not a foolproof method of diagnosis, but because so many other indicators have turned out to not be reliable in diagnosing VZV infections in the CNS, screening for VZV by PCR is recommended. Negative PCR does not rule out VZV involvement, but a positive PCR can be used for diagnosis, and appropriate treatment started (for example, antivirals can be prescribed rather than antibiotics). [10]
The introduction of DNA analysis techniques has shown some complications of varicella-zoster to be more common than previously thought. For example, sporadic meningoencephalitis (ME) caused by varicella-zoster was regarded as a rare disease, mostly related to childhood chickenpox. However, meningoencephalitis caused by varicella-zoster is increasingly recognized as a predominant cause of ME among immunocompetent adults in non-epidemic circumstances.[11]
Diagnosis of complications of varicella-zoster, particularly in cases where the disease reactivates after years or decades of latency, are difficult. A rash (shingles) can be present or absent. Symptoms vary, and there is significant overlap in symptoms with herpes-simplex symptoms.[11]
Although DNA analysis techniques such as Polymerase Chain Reaction can be used to look for DNA of herpesviruses in spinal fluid or blood, the results may be negative, even in cases where other definitive symptoms exist.[12] Notwithstanding these limitations, the use of PCR has resulted in an advance in the state of the art in our understanding of herpesviruses, including VZV, during the 1990s and 2000s. For example, in the past, clinicians believed that encephalitis was caused by herpes simplex and that patients always died or developed serious long term function problems. People were diagnosed at autopsy or by brain biopsy. Brain biopsy is not undertaken lightly: it is reserved only for serious cases that cannot be diagnosed by less invasive methods. For this reason, knowledge of these herpes virus conditions was limited to severe cases. DNA techniques have made it possible to diagnose “mild” cases, caused by VZV or HSV, in which the symptoms include fever, headache, and altered mental status. Mortality rates in treated patients are decreasing.[11]
Morphology
VZV is closely related to the herpes simplex viruses (HSV), sharing much genome homology. The known envelope glycoproteins (gB, gC, gE, gH, gI, gK, gL) correspond with those in HSV; however, there is no equivalent of HSV gD. VZV also fails to produce the LAT (latency-associated transcripts) that play an important role in establishing HSV latency (herpes simplex virus). VZV virions are spherical and 180–200 nm in diameter. Their lipid envelope encloses the 100 nm nucleocapsid of 162 hexameric and pentameric capsomers arranged in an icosahedral form. Its DNA is a single, linear, double-stranded molecule, 125,000 nt long. The capsid is surrounded by a number of loosely associated proteins known collectively as the tegument; many of these proteins play critical roles in initiating the process of virus reproduction in the infected cell. The tegument is in turn covered by a lipid envelope studded with glycoproteins that are displayed on the exterior of the virion, each approximately 8 nm long.
Genome
The genome was first sequenced in 1986.[13] It is a linear duplex DNA molecule, a laboratory strain has 124,884 base pairs. The genome has 2 predominant isomers, depending on the orientation of the S segment, P (prototype) and IS (inverted S) which are present with equal frequency for a total frequency of 90-95%. The L segment can also be inverted resulting in a total of four linear isomers (IL and ILS). This is distinct from HSV's equiprobable distribution, and the discriminatory mechanism is not known. A small percentage of isolated molecules are circular genomes, about which little is known. (It is known that HSV circularizes on infection.) There are at least 70 open reading frames in the genome.
There are at least five clades of this virus.[14] Clades 1 and 3 include European/North American strains; clade 2 are Asian strains, especially from Japan; and clade 5 appears to be based in India. Clade 4 includes some strains from Europe but its geographic origins need further clarification.
Evolution
Commonality with HSV1 and HSV2 indicates a common ancestor, five genes do not have corresponding HSV genes. Relation with other human herpes viruses is less strong, but many homologous and conserved gene blocks are still found.
There are five principal clades (1-5) and four genotypes that do not fit into these clades.[15] The current distribution of these clades is Asia (clades 1, 2, and 5) and Europe (clades 1, 3 and 4). Allocation of VZV strains to clades required a sequence of the whole virus genome. Practically all molecular epidemiological data on global VZV strain distribution obtained with the targeted sequencing of selected regions.
Phylogenetic analysis of VZV genomic sequences resolves wild-type strains into 9 genotypes (E1, E2, J, M1, M2, M3, M4, VIII and IX).[16][17] Complete sequences for M3 and M4 strains are unavailable, but targeted analyses of representative strains suggest they are stable, circulating VZV genotypes. Sequence analysis of VZV isolates identified both shared and specific markers for every genotype and validated a unified VZV genotyping strategy. Despite high genotype diversity no evidence for intra-genotypic recombination was observed. Five of seven VZV genotypes were reliably discriminated using only four single nucleotide polymorphisms (SNP) present in ORF22, and the E1 and E2 genotypes were resolved using SNP located in ORF21, ORF22 or ORF50. Sequence analysis of 342 clinical varicella and zoster specimens from 18 European countries identified the following distribution of VZV genotypes: E1, 221 (65%); E2, 87 (25%); M1, 20 (6%); M2, 3 (1%); M4, 11 (3%). No M3 or J strains were observed.[16] Of 165 clinical varicella and zoster isolates from Australia and New Zealand typed using this approach, 67 of 127 eastern Australian isolates were E1, 30 were E2, 16 were J, 10 were M1, and 4 were M2; 25 of 38 New Zealand isolates were E1, 8 were E2, and 5 were M1.[18]
The mutation rate for synonymous and nonsynonymous mutation rates among the herpesviruses have been estimated at 1 × 10−7 and 2.7 × 10−8 mutations/site/year, respectively, based on the highly conserved gB gene.[19]
Vaccination
A live attenuated VZV Oka/Merck strain vaccine is available and is marketed in the United States under the trade name Varivax. It was developed by Merck, Sharp & Dohme in the 1980s from the Oka strain virus isolated and attenuated by Michiaki Takahashi and colleagues in the 1970s. It was submitted to the US Food and Drug Administration for approval in 1990 and was approved in 1995. Since then, it has been added to the recommended vaccination schedules for children in Australia, the United States, and many other countries. Varicella vaccination has raised concerns in some that the immunity induced by the vaccine may not be lifelong, possibly leaving adults vulnerable to more severe disease as the immunity from their childhood immunization wanes. Vaccine coverage in the United States in the population recommended for vaccination is approaching 90%, with concomitant reductions in the incidence of varicella cases and hospitalizations and deaths due to VZV. So far, clinical data has proved that the vaccine is effective for over 10 years in preventing varicella infection in healthy individuals and when breakthrough infections do occur, illness is typically mild.[20] In 2007, the ACIP recommended a second dose of vaccine before school entry to ensure the maintenance of high levels of varicella immunity.[21]
In 2006, the United States Food and Drug Administration approved Zostavax for the prevention of shingles. Zostavax is a more concentrated formulation of the Varivax vaccine, designed to elicit an immune response in older adults whose immunity to VZV wanes with advancing age. A systematic review by the Cochrane Library shows that Zostavax reduces the incidence of shingles by almost 50%.[22]
See also
Gallery
-
Transmission electron micrograph of varicella-zoster virions from vesicle fluid of patient with chickenpox. From Public Health Image Library (PHIL). [23]
-
Electron micrographs of Variola, Varicella and Vaccinia virions. From Public Health Image Library (PHIL). [23]
-
Transmission electron micrograph (TEM) of a Varicella (Chickenpox) Virus. From Public Health Image Library (PHIL). [23]
-
Various viruses from the Herpesviridae family seen using an electron micrograph. From Public Health Image Library (PHIL). [23]
-
photomicrograph reveals some of the cytoarchitectural histopathologic changes which you’d find in a human skin tissue specimen that included a chickenpox, or varicella zoster virus lesion (500x mag). From Public Health Image Library (PHIL). [23]
-
Hematoxylin-eosin (H&E)-stained photomicrograph reveals some of the cytoarchitectural histopathologic changes found in a human skin tissue specimen that included a varicella zoster virus lesion (50x mag). From Public Health Image Library (PHIL). [23]
-
Hematoxylin-eosin (H&E)-stained photomicrograph reveals some of the cytoarchitectural histopathologic changes found in a human skin tissue specimen that included a varicella zoster virus lesion (50x mag). From Public Health Image Library (PHIL). [23]
-
Hematoxylin-eosin (H&E)-stained photomicrograph reveals some of the cytoarchitectural histopathologic changes found in a human skin tissue specimen that included a varicella zoster virus lesion (500x mag). From Public Health Image Library (PHIL). [23]
-
Hematoxylin-eosin (H&E)-stained photomicrograph reveals some of the cytoarchitectural histopathologic changes found in a human skin tissue specimen that included a varicella zoster virus lesion (1200x mag). From Public Health Image Library (PHIL). [23]
-
Hematoxylin-eosin (H&E)-stained photomicrograph reveals some of the cytoarchitectural histopathologic changes found in a human skin tissue specimen that included a varicella zoster virus lesion (1200x mag). From Public Health Image Library (PHIL). [23]
-
This image depicts three mounted chickenpox scabs seen from the side. From Public Health Image Library (PHIL). [23]
-
Back of boy who had manifested the maculopapular rash that was determined to be chickenpox, also known as varicella-zoster virus (VZV). From Public Health Image Library (PHIL). [23]
-
Chickenpox lesions on the skin of this patient's left breast and arm on day 6 of the illness. From Public Health Image Library (PHIL). [23]
-
Chickenpox lesions on the skin of this patient's back and buttocks at day 6 of the illness. From Public Health Image Library (PHIL). [23]
-
Chickenpox lesions on the skin of this patient's breasts, arms, and torso at day 6 of the illness. From Public Health Image Library (PHIL). [23]
-
Patient with cervical skin lesions caused by chickenpox. From Public Health Image Library (PHIL). [23]
-
4-month old infant with skin lesions on his brow ridge due to chickenpox. From Public Health Image Library (PHIL). [23]
-
Patient had presented with chickenpox demonstrating the typical rash on day eight. From Public Health Image Library (PHIL). [23]
-
Patient developed palatal mucosal lesions due to chickenpox. From Public Health Image Library (PHIL). [23]
-
Vaccine recipient developed a secondary herpes infection adjacent to the vaccination site. From Public Health Image Library (PHIL). [23]
-
Pustulovesicular rash represents a generalized herpes outbreak due to the Varicella-zoster virus (VZV) pathogen. From Public Health Image Library (PHIL). [23]
-
Case of chickenpox. From Public Health Image Library (PHIL). [23]
-
Case of chickenpox. From Public Health Image Library (PHIL). [23]
-
Case of chickenpox. From Public Health Image Library (PHIL). [23]
-
Chickenpox lesions on a patient’s back, which were displaying the characteristic “cropping” distribution, or manifesting themselves in clusters. From Public Health Image Library (PHIL). [23]
-
Posterior view of a hospitalized man's neck, back and shoulders, who’d been assigned a bed in a smallpox ward, due to an initially misdiagnosed illness, which turned out to be chickenpox. From Public Health Image Library (PHIL). [23]
-
View of a patient’s thighs and upper legs, who’d been diagnosed with chickenpox. From Public Health Image Library (PHIL). [23]
-
Pathologic changes seen on the surface of the right unilateral side of this elderly male patient’s tongue and chin, represent a herpes outbreak due to the Varicella-zoster virus (VZV) pathogen. From Public Health Image Library (PHIL). [23]
-
Viewed from above, this image depicts a smallpox scab (left), and chickenpox scab (right) as a demonstration in comparative morphology. From Public Health Image Library (PHIL). [23]
-
Close-up of a maculopapular rash that was diagnosed as a crop of chickenpox lesions. From Public Health Image Library (PHIL). [23]
-
Lateral view of a 4 month-old infant’s face with a single varicella-zoster, otherwise known as chickenpox. From Public Health Image Library (PHIL). [23]
-
This anteroposterior (AP) radiograph revealed bilateral pulmonary infiltrates throughout the entirety of each lung field in the case of a child with leukemia, as well as chickenpox pneumonia. From Public Health Image Library (PHIL). [23]
-
Image depicts three mounted chickenpox scabs seen from the side revealing the superficiality of these scabs when morphologically compared to a smallpox scab. From Public Health Image Library (PHIL). [23]
-
Volar surface of a patient’s left forearm, including the palmar surface of the left hand upon which you’ll note classic maculopapular rash of chickenpox. From Public Health Image Library (PHIL). [23]
-
Right lateral surface of a patient’s right lower leg and foot with classic maculopapular rash of chickenpox. From Public Health Image Library (PHIL). [23]
-
Bilateral pulmonary infiltrates throughout the entirety of each lung field in the case of a child with leukemia, as well as chickenpox pneumonia. From Public Health Image Library (PHIL). [23]
References
- ↑ "The protean neurologic manifestations of varicella-zoster virus infection". Cleveland Clinic Journal of Medicine. July 2007.
- ↑ Steiner I (2007). "The neurotropic herpes viruses: herpes simplex and varicella-zoster". Lancet Neurol. 6 (11): 1015–28. doi:10.1016/S1474-4422(07)70267-3. PMID 17945155. Unknown parameter
|coauthors=ignored (help) - ↑ "Infection of the central nervous system caused by varicella zoster virus reactivation: a retrospective case series study". International Journal of Infectious Diseases. July 2013.
- ↑ "Mapping amyotrophic lateral sclerosis lake risk factors across northern New England". International Journal of Health Geographics. 2014.
- ↑ "The varicella zoster virus vasculopathies: clinical, CSF, imaging, and virologic features". Neurology. March 2008.
- ↑ "Infection of the central nervous system caused by varicella zoster virus reactivation: a retrospective case series study". International Journal of Infectious Diseases. July 2013.
- ↑ "Infection of the central nervous system caused by varicella zoster virus reactivation: a retrospective case series study". International Journal of Infectious Diseases. July 2013.
- ↑ "Clinical Features of Viral Meningitis in Adults: Significant Differences in Cerebrospinal Fluid Findings among Herpes Simplex Virus, Varicella Zoster Virus, and Enterovirus Infections" (PDF). Clinical Infectious Diseases, the Infectious Diseases Society of America. 2008.
- ↑ "Infection of the central nervous system caused by varicella zoster virus reactivation: a retrospective case series study". International Journal of Infectious Diseases. July 2013.
- ↑ "Infection of the central nervous system caused by varicella zoster virus reactivation: a retrospective case series study". International Journal of Infectious Diseases. July 2013.
- ↑ 11.0 11.1 11.2 "Varicella zoster vs. herpes simplex meningoencephalitis in the PCR era. A single center study". Journal of the Neurological Sciences. August 2011.
- ↑ "Recurrent Herpes Simplex Virus Type 2 Meningitis: A Case Report of Mollaret's Meningitis" (PDF). Jpn. J. Infect. Dis. July 2002.
- ↑ Davison AJ, Scott JE (1986) The complete DNA sequence of varicella-zoster virus. J Gen Virol 67:1759-1816
- ↑ Template:Cite doi
- ↑ Template:Cite doi
- ↑ 16.0 16.1 PMID 19019403 (PMID 19019403)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Template:Cite doi
- ↑ Template:Cite doi
- ↑ McGeoch DJ, Cook S (1994) Molecular phylogeny of the alphaherpesvirinae subfamily and a proposed evolutionary timescale. J Mol Biol 238:9-22
- ↑ "Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers for Disease Control and Prevention". MMWR Recomm Rep. 45 (RR–11): 1–36. July 1996. PMID 8668119.
- ↑ Marin M (June 2007). "Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP)". MMWR Recomm Rep. 56 (RR–4): 1–40. PMID 17585291. Unknown parameter
|coauthors=ignored (help) - ↑ Gagliardi AM, Gomes Silva BN, Torloni MR, Soares BG (2012). Gagliardi, Anna MZ, ed. "Vaccines for preventing herpes zoster in older adults". Cochrane Database Syst Rev. 10: CD008858. doi:10.1002/14651858.CD008858.pub2. PMID 23076951.
- ↑ 23.00 23.01 23.02 23.03 23.04 23.05 23.06 23.07 23.08 23.09 23.10 23.11 23.12 23.13 23.14 23.15 23.16 23.17 23.18 23.19 23.20 23.21 23.22 23.23 23.24 23.25 23.26 23.27 23.28 23.29 23.30 23.31 23.32 23.33 23.34 23.35 23.36 "Public Health Image Library (PHIL)".
External links
- "Varicella (Chickenpox) Vaccination" Centers for Disease Control and Prevention (CDC)
![Transmission electron micrograph of varicella-zoster virions from vesicle fluid of patient with chickenpox. From Public Health Image Library (PHIL). [23]](/images/b/b7/VZV15.jpeg)
![Electron micrographs of Variola, Varicella and Vaccinia virions. From Public Health Image Library (PHIL). [23]](/images/a/a3/Chickenpox38.jpeg)
![Transmission electron micrograph (TEM) of a Varicella (Chickenpox) Virus. From Public Health Image Library (PHIL). [23]](/images/f/fa/VZV14.jpeg)
![Various viruses from the Herpesviridae family seen using an electron micrograph. From Public Health Image Library (PHIL). [23]](/images/7/76/VZV13.jpeg)
![photomicrograph reveals some of the cytoarchitectural histopathologic changes which you’d find in a human skin tissue specimen that included a chickenpox, or varicella zoster virus lesion (500x mag). From Public Health Image Library (PHIL). [23]](/images/e/e2/VZV09.jpeg)
![Hematoxylin-eosin (H&E)-stained photomicrograph reveals some of the cytoarchitectural histopathologic changes found in a human skin tissue specimen that included a varicella zoster virus lesion (50x mag). From Public Health Image Library (PHIL). [23]](/images/f/fa/VZV08.jpeg)
![Hematoxylin-eosin (H&E)-stained photomicrograph reveals some of the cytoarchitectural histopathologic changes found in a human skin tissue specimen that included a varicella zoster virus lesion (50x mag). From Public Health Image Library (PHIL). [23]](/images/3/3d/VZV07.jpeg)
![Hematoxylin-eosin (H&E)-stained photomicrograph reveals some of the cytoarchitectural histopathologic changes found in a human skin tissue specimen that included a varicella zoster virus lesion (500x mag). From Public Health Image Library (PHIL). [23]](/images/3/3d/VZV06.jpeg)
![Hematoxylin-eosin (H&E)-stained photomicrograph reveals some of the cytoarchitectural histopathologic changes found in a human skin tissue specimen that included a varicella zoster virus lesion (1200x mag). From Public Health Image Library (PHIL). [23]](/images/1/14/VZV05.jpeg)
![Hematoxylin-eosin (H&E)-stained photomicrograph reveals some of the cytoarchitectural histopathologic changes found in a human skin tissue specimen that included a varicella zoster virus lesion (1200x mag). From Public Health Image Library (PHIL). [23]](/images/8/84/VZV04.jpeg)
![From Public Health Image Library (PHIL). [23]](/images/7/72/VZV03.jpeg)
![This image depicts three mounted chickenpox scabs seen from the side. From Public Health Image Library (PHIL). [23]](/images/1/1f/VZV02.jpeg)
![Back of boy who had manifested the maculopapular rash that was determined to be chickenpox, also known as varicella-zoster virus (VZV). From Public Health Image Library (PHIL). [23]](/images/f/fd/VZV01.jpeg)
![Chickenpox lesions on the skin of this patient's left breast and arm on day 6 of the illness. From Public Health Image Library (PHIL). [23]](/images/9/95/Chickenpox36.jpeg)
![Chickenpox lesions on the skin of this patient's back and buttocks at day 6 of the illness. From Public Health Image Library (PHIL). [23]](/images/8/8e/Chickenpox35.jpeg)
![Chickenpox lesions on the skin of this patient's breasts, arms, and torso at day 6 of the illness. From Public Health Image Library (PHIL). [23]](/images/7/71/Chickenpox34.jpeg)
![Patient with cervical skin lesions caused by chickenpox. From Public Health Image Library (PHIL). [23]](/images/c/c7/Chickenpox33.jpeg)
![4-month old infant with skin lesions on his brow ridge due to chickenpox. From Public Health Image Library (PHIL). [23]](/images/0/03/Chickenpox32.jpeg)
![Patient had presented with chickenpox demonstrating the typical rash on day eight. From Public Health Image Library (PHIL). [23]](/images/8/84/Chickenpox29.jpeg)
![Patient developed palatal mucosal lesions due to chickenpox. From Public Health Image Library (PHIL). [23]](/images/1/18/Chickenpox28.jpeg)
![Vaccine recipient developed a secondary herpes infection adjacent to the vaccination site. From Public Health Image Library (PHIL). [23]](/images/3/3e/Chickenpox27.jpeg)
![Pustulovesicular rash represents a generalized herpes outbreak due to the Varicella-zoster virus (VZV) pathogen. From Public Health Image Library (PHIL). [23]](/images/6/6e/Chickenpox26.jpeg)
![Case of chickenpox. From Public Health Image Library (PHIL). [23]](/images/e/e2/Chickenpox24.jpeg)
![Case of chickenpox. From Public Health Image Library (PHIL). [23]](/images/6/6c/Chickenpox23.jpeg)
![Case of chickenpox. From Public Health Image Library (PHIL). [23]](/images/5/5a/Chickenpox22.jpeg)
![Chickenpox lesions on a patient’s back, which were displaying the characteristic “cropping” distribution, or manifesting themselves in clusters. From Public Health Image Library (PHIL). [23]](/images/4/47/Chickenpox21.jpeg)
![Posterior view of a hospitalized man's neck, back and shoulders, who’d been assigned a bed in a smallpox ward, due to an initially misdiagnosed illness, which turned out to be chickenpox. From Public Health Image Library (PHIL). [23]](/images/e/e9/Chickenpox20.jpeg)
![View of a patient’s thighs and upper legs, who’d been diagnosed with chickenpox. From Public Health Image Library (PHIL). [23]](/images/2/21/Chickenpox19.jpeg)
![Pathologic changes seen on the surface of the right unilateral side of this elderly male patient’s tongue and chin, represent a herpes outbreak due to the Varicella-zoster virus (VZV) pathogen. From Public Health Image Library (PHIL). [23]](/images/b/b8/Chickenpox18.jpeg)
![Viewed from above, this image depicts a smallpox scab (left), and chickenpox scab (right) as a demonstration in comparative morphology. From Public Health Image Library (PHIL). [23]](/images/a/ae/Chickenpox17.jpeg)
![Close-up of a maculopapular rash that was diagnosed as a crop of chickenpox lesions. From Public Health Image Library (PHIL). [23]](/images/a/a8/Chickenpox16.jpeg)
![This anteroposterior (AP) radiograph revealed bilateral pulmonary infiltrates throughout the entirety of each lung field in the case of a child with leukemia, as well as chickenpox pneumonia. From Public Health Image Library (PHIL). [23]](/images/0/0f/Chickenpox06.jpeg)
![Image depicts three mounted chickenpox scabs seen from the side revealing the superficiality of these scabs when morphologically compared to a smallpox scab. From Public Health Image Library (PHIL). [23]](/images/0/09/Chickenpox04.jpeg)
![Volar surface of a patient’s left forearm, including the palmar surface of the left hand upon which you’ll note classic maculopapular rash of chickenpox. From Public Health Image Library (PHIL). [23]](/images/0/09/Chickenpox03.jpeg)
![Right lateral surface of a patient’s right lower leg and foot with classic maculopapular rash of chickenpox. From Public Health Image Library (PHIL). [23]](/images/5/50/Chickenpox02.jpeg)
![Bilateral pulmonary infiltrates throughout the entirety of each lung field in the case of a child with leukemia, as well as chickenpox pneumonia. From Public Health Image Library (PHIL). [23]](/images/5/55/Chickenpox05.jpeg)