Umeclidinium
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Umeclidinium is a respiratory agent that is FDA approved for the treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema. Common adverse reactions include nasopharyngitis, upper respiratory infection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Chronic obstructive pulmonary disease
- Umeclidinium is an anticholinergic indicated for the long-term, once-daily, maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
Dosing Information
- Umeclidinium(umeclidinium 62.5 mcg) should be administered as 1 inhalation once daily by the orally inhaled route only.
- Umeclidiniumshould be taken at the same time every day. Do not use Umeclidinium more than 1 time every 24 hours.
- No dosage adjustment is required for geriatric patients, patients with renal impairment, or patients with moderate hepatic impairment
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Umeclidinium in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Umeclidinium in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Umeclidinium in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Umeclidinium in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Umeclidinium in pediatric patients.
Contraindications
- The use of Umeclidinium is contraindicated in the following conditions:
- Severe hypersensitivity to milk proteins
- Hypersensitivity to umeclidinium or any of the excipients
Warnings
Deterioration of Disease and Acute Episodes
- Umeclidinium should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of COPD. Umeclidiniumh as not been studied in subjects with acutely deteriorating COPD. *The initiation of Umeclidinium in this setting is not appropriate.
- Umeclidinium should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. Umeclidiniumhas not been studied in the relief of acute symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist.
- COPD may deteriorate acutely over a period of hours or chronically over several days or longer. If Umeclidiniumno longer controls symptoms of bronchoconstriction; the patient’s inhaled, short-acting beta2-agonist becomes less effective; or the patient needs more short-acting beta2-agonist than usual, these may be markers of deterioration of disease. In this setting a re-evaluation of the patient and the COPD treatment regimen should be undertaken at once. Increasing the daily dose of Umeclidinium beyond the recommended dose is not appropriate in this situation.
Paradoxical Bronchospasm
- As with other inhaled medicines, Umeclidinium can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs following dosing with Umeclidinium, it should be treated immediately with an inhaled, short-acting bronchodilator; Umeclidinium should be discontinued immediately; and alternative therapy should be instituted.
Hypersensitivity Reactions
- Hypersensitivity reactions may occur after administration of Umeclidinium. There have been reports of anaphylactic reactions in patients with severe milk protein allergy after inhalation of other powder products containing lactose; therefore, patients with severe milk protein allergy should not use Umeclidinium.
Worsening of Narrow-Angle Glaucoma
- Umeclidinium should be used with caution in patients with narrow-angle glaucoma. Prescribers and patients should be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a physician immediately if any of these signs or symptoms develops.
Worsening of Urinary Retention
- Umeclidinium should be used with caution in patients with urinary retention. Prescribers and patients should be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, painful urination), especially in patients with prostatic hyperplasia or bladder-neck obstruction. Instruct patients to consult a physician immediately if any of these signs or symptoms develops.
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions are described in greater detail in other sections:
- Paradoxical bronchospasm
- Worsening of narrow-angle glaucoma
- Worsening of urinary retention
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- A total of 1,663 subjects with COPD across 8 clinical trials (mean age: 62.7 years; 89% white; 65% male across all treatments, including placebo) received at least 1 inhalation dose of umeclidinium at doses of 62.5 or 125 mcg. In the 4 randomized, double-blind, placebo- or active-controlled, efficacy clinical trials, 1,185 subjects received umeclidinium for up to 24 weeks, of which 487 subjects received the recommended dose of umeclidinium 62.5 mcg. In a 12-month, randomized, double-blind, placebo-controlled, long-term safety trial, 227 subjects received umeclidinium 125 mcg for up to 52 weeks.
- The incidence of adverse reactions associated with Umeclidinium in Table 1 is based upon 2 placebo-controlled efficacy trials: one 12-week trial and one 24-week trial.
- Other adverse reactions with Umeclidinium observed with an incidence less than 1% but more common than placebo included atrial fibrillation.
- In a long-term safety trial, 336 subjects (n = 227 umeclidinium 125 mcg, n = 109 placebo) were treated for up to 52 weeks with umeclidinium 125 mcg or placebo. The demographic and baseline characteristics of the long-term safety trial were similar to those of the efficacy trials described above. Adverse reactions that occurred with a frequency greater than or equal to 1% in subjects receiving umeclidinium 125 mcg that exceeded that in placebo in this trial were: nasopharyngitis, upper respiratory tract infection, urinary tract infection, pharyngitis, pneumonia, lower respiratory tract infection, rhinitis, supraventricular tachycardia, supraventricular extrasystoles, sinus tachycardia, idioventricular rhythm, headache, dizziness, sinus headache, cough, back pain, arthralgia, pain in extremity, neck pain, myalgia, nausea, dyspepsia, diarrhea, rash, depression, and vertigo.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Umeclidinium in the drug label.
Drug Interactions
Anticholinergics
- There is potential for an additive interaction with concomitantly used anticholinergic medicines. Therefore, avoid coadministration of Umeclidiniumwith other anticholinergic-containing drugs as this may lead to an increase in anticholinergic adverse effects
Use in Specific Populations
Pregnancy
- Teratogenic Effects:There are no adequate and well-controlled trials with Umeclidinium in pregnant women. Because animal reproduction studies are not always predictive of human response, Umeclidinium should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Women should be advised to contact their physicians if they become pregnant while taking Umeclidinium.
- There was no evidence of teratogenic effects in rats and rabbits at approximately 50 and 200 times, respectively, the MRHDID (maximum recommended human daily inhaled dose) in adults (on an AUC basis at maternal inhaled doses up to 278 mcg/kg/day in rats and maternal subcutaneous doses up to 180 mcg/kg/day in rabbits).
- Nonteratogenic Effects: There were no effects on perinatal and postnatal developments in rats at approximately 80 times the MRHDID in adults (on an AUC basis at maternal subcutaneous doses up to 180 mcg/kg/day).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Umeclidinium in women who are pregnant.
Labor and Delivery
- There are no adequate and well-controlled human trials that have investigated the effects of Umeclidinium during labor and delivery. Umeclidinium should be used during labor only if the potential benefit justifies the potential risk.
Nursing Mothers
- It is not known whether Umeclidinium is excreted in human breast milk. Because many drugs are excreted in human milk, caution should be exercised when Umeclidinium is administered to a nursing woman. Since there are no data from well-controlled human studies on the use of Umeclidinium by nursing mothers, a decision should be made whether to discontinue nursing or to discontinue Umeclidinium,taking into account the importance of Umeclidinium to the mother.
- Subcutaneous administration of umeclidinium to lactating rats at approximately 25 times the MRHDID in adults resulted in a quantifiable level of umeclidinium in 2 pups, which may indicate transfer of umeclidinium in milk.
Pediatric Use
- Umeclidinium is not indicated for use in children. The safety and efficacy in pediatric patients have not been established.
Geriatic Use
- Based on available data, no adjustment of the dosage of Umeclidinium in geriatric patients is necessary, but greater sensitivity in some older individuals cannot be ruled out.
- Clinical trials of Umeclidiniumincluded 810 subjects aged 65 years and older, and, of those, 183 subjects were aged 75 years and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger subjects.
Gender
There is no FDA guidance on the use of Umeclidinium with respect to specific gender populations.
Race
There is no FDA guidance on the use of Umeclidinium with respect to specific racial populations.
Renal Impairment
- Patients with severe renal impairment (creatinine clearance less than 30 mL/min) showed no relevant increases in Cmax or AUC, nor did protein binding differ between subjects with severe renal impairment and their healthy controls. No dosage adjustment is required in patients with renal impairment
Hepatic Impairment
- Patients with moderate hepatic impairment (Child-Pugh score of 7-9) showed no relevant increases in Cmax or AUC, nor did protein binding differ between subjects with moderate hepatic impairment and their healthy controls. Studies in subjects with severe hepatic impairment have not been performed
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Umeclidinium in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Umeclidinium in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Inhalation
Monitoring
There is limited information regarding Monitoring of Umeclidinium in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Umeclidinium in the drug label.
Overdosage
- No case of overdose has been reported with umeclidinium.
- High doses of umeclidinium may lead to anticholinergic signs and symptoms. However, there were no systemic anticholinergic adverse effects following a once-daily inhaled dose of up to 1,000 mcg umeclidinium (16 times the maximum recommended daily dose) for 14 days in subjects with COPD.
- Treatment of overdosage consists of discontinuation of Umeclidinium together with institution of appropriate symptomatic and/or supportive therapy.
Pharmacology
| |
Umeclidinium bromide
| |
| Systematic (IUPAC) name | |
| Diphenyl-[1-(2-phenylmethoxyethyl)-1-azoniabicyclo[2.2.2]octan-4-yl]methanol bromide | |
| Identifiers | |
| CAS number | |
| ATC code | R03 R03AL03 (WHO) (+vilanterol) |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 508.49 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
- Umeclidinium is a long-acting, antimuscarinic agent, which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors M1 to M5. In the airways, it exhibits pharmacological effects through the inhibition of M3 receptor at the smooth muscle leading to bronchodilation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In preclinical in vitro as well as in vivostudies, prevention of methacholine and acetylcholine-induced bronchoconstrictive effects was dose-dependent and lasted longer than 24 hours. The clinical relevance of these findings is unknown. The bronchodilation following inhalation of umeclidinium is predominantly a site-specific effect.
Structure
- Umeclidiniumcontains the active ingredient umeclidinium, an anticholinergic.
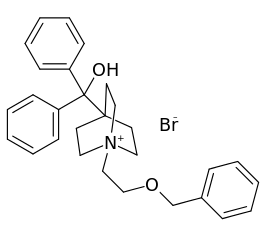
Umeclidinium bromide has the chemical name 1-[2-(benzyloxy)ethyl]-4-(hydroxydiphenylmethyl)-1-azoniabicyclo[2.2.2]octane bromide and the following chemical structure:
- Umeclidinium bromide is a white powder with a molecular weight of 508.5, and the empirical formula is C29H34NO2•Br (as a quaternary ammonium bromide compound). It is slightly soluble in water.
- Umeclidinium is a light grey and light green plastic inhaler containing a double-foil blister strip. Each blister on the strip contains a white powder mix of micronized umeclidinium bromide (74.2 mcg equivalent to 62.5 mcg of umeclidinium), magnesium stearate (75 mcg), and lactose monohydrate (to 12.5 mg). The lactose monohydrate contains milk proteins. After the inhaler is activated, the powder within the blister is exposed and ready for dispersion into the airstream created by the patient inhaling through the mouthpiece.
- Under standardized in vitro test conditions, Umeclidinium delivers 55 mcg of umeclidinium per dose when tested at a flow rate of 60 L/min for 4 seconds.
- In adult subjects with obstructive lung disease and severely compromised lung function (COPD with forced expiratory volume in 1 second/forced vital capacity [FEV1/FVC] less than 70% and FEV1 less than 30% predicted or FEV1 less than 50% predicted plus chronic respiratory failure), mean peak inspiratory flow through the ELLIPTA inhaler was 67.5 L/min (range: 41.6 to 83.3 L/min).
- The actual amount of drug delivered to the lung will depend on patient factors, such as inspiratory flow profile.
Pharmacodynamics
- Cardiac Electrophysiology: QTc interval prolongation was studied in a double-blind, multiple dose, placebo- and positive-controlled, crossover trial in 86 healthy subjects. Following repeat doses of umeclidinium 500 mcg once daily (8 times the recommended dosage) for 10 days, umeclidinium does not prolong QTc to any clinically relevant extent.
Pharmacokinetics
- Linear pharmacokinetics was observed for umeclidinium (62.5 to 500 mcg).
- Absorption: Umeclidinium plasma levels may not predict therapeutic effect. Following inhaled administration of umeclidinium in healthy subjects, Cmax occurred at 5 to 15 minutes. Umeclidinium is mostly absorbed from the lung after inhaled doses with minimum contribution from oral absorption. Following repeat dosing of inhaled umeclidinium, steady state was achieved within 14 days with 1.8-fold accumulation.
- Distribution: Following intravenous administration to healthy subjects, the mean volume of distribution was 86 L. In vitro plasma protein binding in human plasma was on average 89%.
- Metabolism: In vitro data showed that umeclidinium is primarily metabolized by the enzyme cytochrome P450 2D6 (CYP2D6) and is a substrate for the P-glycoprotein (P-gp) transporter. The primary metabolic routes for umeclidinium are oxidative (hydroxylation, O-dealkylation) followed by conjugation (e.g., glucuronidation), resulting in a range of metabolites with either reduced pharmacological activity or for which the pharmacological activity has not been established. Systemic exposure to the metabolites is low.
- Elimination: Following intravenous dosing with radiolabeled umeclidinium, mass balance showed 58% of the radiolabel in the feces and 22% in the urine. The excretion of the drug-related material in the feces following intravenous dosing indicated elimination in the bile. Following oral dosing to healthy male subjects, radiolabel recovered in feces was 92% of the total dose and that in urine was less than 1% of the total dose, suggesting negligible oral absorption. The effective half-life after once daily dosing is 11 hours.
- Special Populations: Population pharmacokinetic analysis showed no evidence of a clinically significant effect of age (40 to 93 years) (see Figure 1), gender (69% male) (see Figure 1), inhaled corticosteroid use (48%), or weight (34 to 161 kg) on systemic exposure of umeclidinium. In addition, there was no evidence of a clinically significant effect of race.
- Hepatic Impairment: The impact of hepatic impairment on the pharmacokinetics of Umeclidinium has been evaluated in subjects with moderate hepatic impairment (Child-Pugh score of 7-9). There was no evidence of an increase in systemic exposure to umeclidinium (Cmax and AUC) (see Figure 1). There was no evidence of altered protein binding in subjects with moderate hepatic impairment compared with healthy subjects. Umeclidinium has not been evaluated in subjects with severe hepatic impairment.
- Renal Impairment: The pharmacokinetics of Umeclidinium has been evaluated in subjects with severe renal impairment (creatinine clearance less than 30 mL/min). There was no evidence of an increase in systemic exposure to umeclidinium (Cmax and AUC) (see Figure 1). There was no evidence of altered protein binding in subjects with severe renal impairment compared with healthy subjects.
- Drug Interactions: Umeclidinium and P-glycoprotein Transporter: Umeclidinium is a substrate of P-gp. The effect of the moderate P-gp transporter inhibitor verapamil (240 mg once daily) on the steady-state pharmacokinetics of umeclidinium was assessed in healthy subjects. No effect on umeclidinium Cmax was observed; however, an approximately 1.4-fold increase in umeclidinium AUC was observed (see Figure 1).
- Umeclidinium and Cytochrome P450 2D6: In vitro metabolism of umeclidinium is mediated primarily by CYP2D6. However, no clinically meaningful difference in systemic exposure to umeclidinium (500 mcg) (8 times the approved dose) was observed following repeat daily inhaled dosing to normal (ultrarapid, extensive, and intermediate metabolizers) and CYP2D6 poor metabolizer subjects (see Figure 1).
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Umeclidinium produced no treatment-relaed increases in the incidence of tumors in 2-year inhalation studies in rats and mice at inhaled doses up to 137 and 295/200 mcg/kg/day (male/female), respectively (approximately 20 and 25/20 times the MRHDID in adults on an AUC basis, respectively).
- Umeclidinium tested negative in the following genotoxicity assays: the in vitro Ames assay, in vitromouse lymphoma assay, and in vivo rat bone marrow micronucleus assay.
- No evidence of impairment of fertility was observed in male and female rats at subcutaneous doses up to 180 mcg/kg/day and inhaled doses up to 294 mcg/kg/day, respectively (approximately 100 and 50 times, respectively, the MRHDID in adults on an AUC basis).
Clinical Studies
- The safety and efficacy of umeclidinium 62.5 mcg were evaluated in 3 dose-ranging trials, 2 placebo-controlled clinical trials (one 12-week trial and one 24-week trial), and a 12-month long-term safety trial. The efficacy of Umeclidinium is based primarily on the dose-ranging trials in 624 subjects with COPD and the 2 placebo-controlled confirmatory trials in 1,738 subjects with COPD.
Dose-Ranging Trials
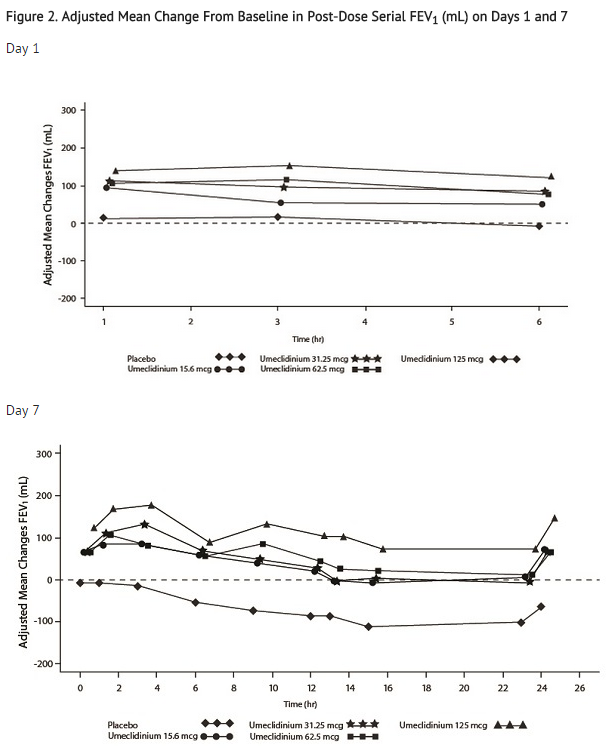
- Dose selection for umeclidinium in COPD was supported by a 7-day, randomized, double-blind, placebo-controlled, crossover trial evaluating 4 doses of umeclidinium (15.6 to 125 mcg) or placebo dosed once daily in the morning in 163 subjects with COPD. A dose ordering was observed, with the 62.5- and 125-mcg doses demonstrating larger improvements in FEV1 over 24 hours compared with the lower doses of 15.6 and 31.25 mcg (Figure 2).
- The differences in trough FEV1 from baseline after 7 days for placebo and the 15.6-, 31.25-, 62.5-, and 125-mcg doses were -74 mL (95% CI: -118, -31), 38 mL (95% CI: -6, 83), 27 mL (95% CI: -18, 72), 49 mL (95% CI: 6, 93), and 109 mL (95% CI: 65, 152), respectively. Two additional dose-ranging trials in subjects with COPD demonstrated minimal additional benefit at doses above 125 mcg. The dose-ranging results supported the evaluation of 2 doses of umeclidinium, 62.5 and 125 mcg, in the confirmatory COPD trials to further assess dose response.
- Evaluations of dosing interval by comparing once- and twice-daily dosing supported selection of a once-daily dosing interval for further evaluation in the confirmatory COPD trials.
Confirmatory Trials
- The clinical development program for Umeclidiniumincluded 2 randomized, double-blind, placebo-controlled, parallel-group trials in subjects with COPD designed to evaluate the efficacy of Umeclidiniumon lung function. Trial 1 was a 24-week placebo-controlled trial, and Trial 2 was a 12-week placebo-controlled trial. These trials treated subjects that had a clinical diagnosis of COPD, were 40 years of age or older, had a history of smoking greater than or equal to 10 pack-years, had a post-albuterol FEV1 less than or equal to 70% of predicted normal values, had a ratio of FEV1/FVC of less than 0.7, and had a Modified Medical Research Council (mMRC) score greater than or equal to 2. Subjects in Trial 1 had a mean age of 63 years and an average smoking history of 46 pack-years, with 50% identified as current smokers. At screening, the mean post-bronchodilator percent predicted FEV1was 47% (range: 13% to 74%), the mean post-bronchodilator FEV1/FVC ratio was 0.47 (range: 0.20 to 0.74), and the mean percent reversibility was 15% (range: -35% to 109%). Baseline demographics and lung function for subjects in Trial 2 were similar to those in Trial 1.
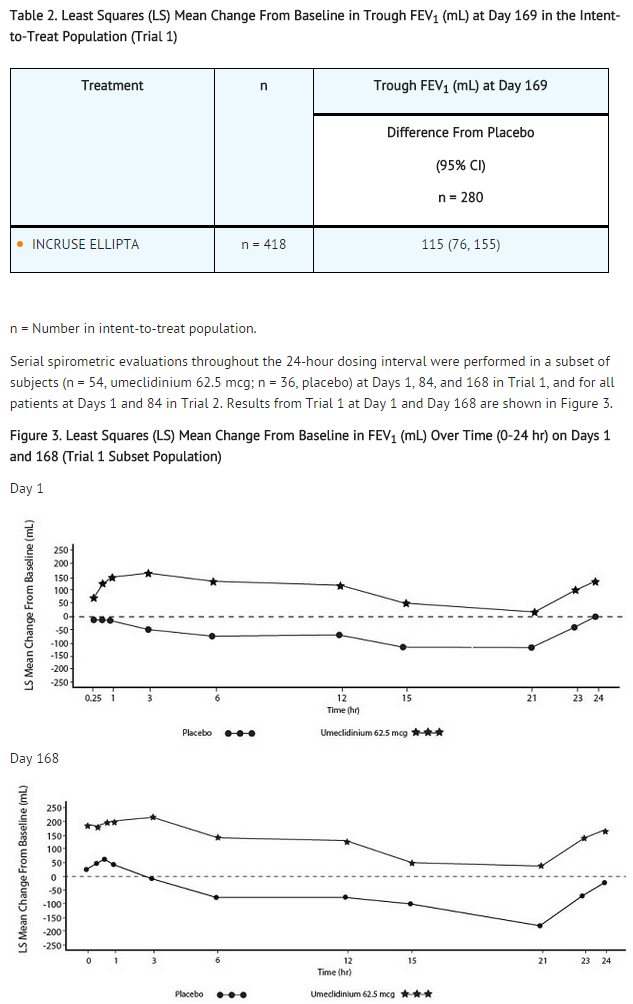
- Trial 1 evaluated umeclidinium 62.5 mcg and placebo. The primary endpoint was change from baseline in trough (predose) FEV1 at Day 169 (defined as the mean of the FEV1 values obtained at 23 and 24 hours after the previous dose on Day 168) compared with placebo. Umeclidinium62.5 mcg demonstrated a larger increase in mean change from baseline in trough (predose) FEV1 relative to placebo (see Table 2). Similar results were obtained from Trial 2.
- In Trial 1, the mean peak FEV1 (over the first 6 hours relative to baseline) at Day 1 and at Day 168 for the group receiving umeclidinium 62.5 mcg compared with placebo was 126 and 130 mL, respectively.
- Health-related quality of life was measured using St. George’s Respiratory Questionnaire (SGRQ). Umeclidinium demonstrated an improvement in mean SGRQ total score compared with placebo treatment at Day 168: -4.69 (95% CI: -7.07,-2.31). The proportion of patients with a clinically meaningful decrease (defined as a decrease of at least 4 units from baseline) at Week 24 was greater for Umeclidinium62.5 mcg (42%; 172/410) compared with placebo (31%; 86/274).
How Supplied
- Umeclidiniumis supplied as a disposable light grey and light green plastic inhaler containing a double-foil blister strip with 30 blisters. The inhaler is packaged in a moisture-protective foil tray with a desiccant and a peelable lid (NDC 0173-0873-10).
- Umeclidiniumis also supplied in an institutional pack of a disposable light grey and light green plastic inhaler containing a double-foil blister strip with 7 blisters. The inhaler is packaged in a moisture-protective foil tray with a desiccant and a peelable lid (NDC 0173-0873-06).
Storage
- Store at room temperature between 68°F and 77°F (20°C and 25°C); excursions permitted from 59°F to 86°F (15°C to 30°C) [See USP Controlled Room Temperature]. Store in a dry place away from direct heat or sunlight. Keep out of reach of children.
- Umeclidiniumshould be stored inside the unopened moisture-protective foil tray and only removed from the tray immediately before initial use. Discard Umeclidinium 6 weeks after opening the foil tray or when the counter reads “0” (after all blisters have been used), whichever comes first. The inhaler is not reusable. Do not attempt to take the inhaler apart.
Images
Drug Images
{{#ask: Page Name::Umeclidinium |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Umeclidinium |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Not for Acute Symptoms: Inform patients that Umeclidiniumis not meant to relieve acute symptoms of COPD and extra doses should not be used for that purpose. Advise them to treat acute symptoms with a rescue inhaler such as albuterol. Provide patients with such medicine and instruct them in how it should be used.
- Instruct patients to seek medical attention immediately if they experience any of the following:
- Symptoms get worse
- Need for more inhalations than usual of their rescue inhaler
- Patients should not stop therapy with Umeclidiniumwithout physician/provider guidance since symptoms may recur after discontinuation.
- Paradoxical Bronchospasm: As with other inhaled medicines, Umeclidinium can cause paradoxical bronchospasm. If paradoxical bronchospasm occurs, instruct patients to discontinue Umeclidinium
- Worsening of Narrow-Angle Glaucoma: Instruct patients to be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a physician immediately if any of these signs or symptoms develops.
- Worsening of Urinary Retention: Instruct patients to be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, painful urination). Instruct patients to consult a physician immediately if any of these signs or symptoms develops.
Precautions with Alcohol
- Alcohol-Umeclidinium interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Incruse Ellipta
Look-Alike Drug Names
There is limited information regarding Umeclidinium Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Umeclidinium |Label Name=Umeclidinium04.png
}}
{{#subobject:
|Label Page=Umeclidinium |Label Name=Umeclidinium05.png
}}