Triosephosphate isomerase
Template:Protbox Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Triose-phosphate isomerase (TPI or TIM), is an enzyme (EC 5.3.1.1) that catalyzes the reversible interconversion of the triose phosphate isomers dihydroxyacetone phosphate and D-glyceraldehyde 3-phosphate.
| Dihydroxyacetone phosphate | {{{forward_enzyme}}} | D-glyceraldehyde 3-phosphate | |
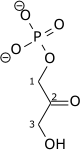
|
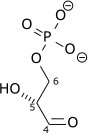
| ||
| {{{minor_forward_substrate(s)}}} | {{{minor_forward_product(s)}}} | ||
| [[image:Biochem_reaction_arrow_{{{reaction_direction_(forward/reversible/reverse)}}}_NNNN_horiz_med.svg|75px]] | |||
| triose phosphate isomerase | |||
Compound C00111 at KEGG Pathway Database.Enzyme 5.3.1.1 at KEGG Pathway Database.Compound C00118 at KEGG Pathway Database.
Triose phosphate isomerase (TPI) plays an important role in glycolysis and is essential for efficient energy production. TPI has been found in nearly every organism searched for the enzyme, including animals such as mammals and insects as well as in fungi, plants and bacteria. However, some bacteria that do not perform glycolysis, like ureaplasmas, lack TPI.
In humans, deficiencies in TPI are associated with a progressive, severe neurological disorder called Triose Phosphate Isomerase deficiency.
Triose phosphate isomerase is a massively efficient enzyme, performing the reaction billions of times faster than it would occur naturally in solution. The reaction is so efficient it is limited only by the rate the substrate can diffuse into the enzyme's active site.
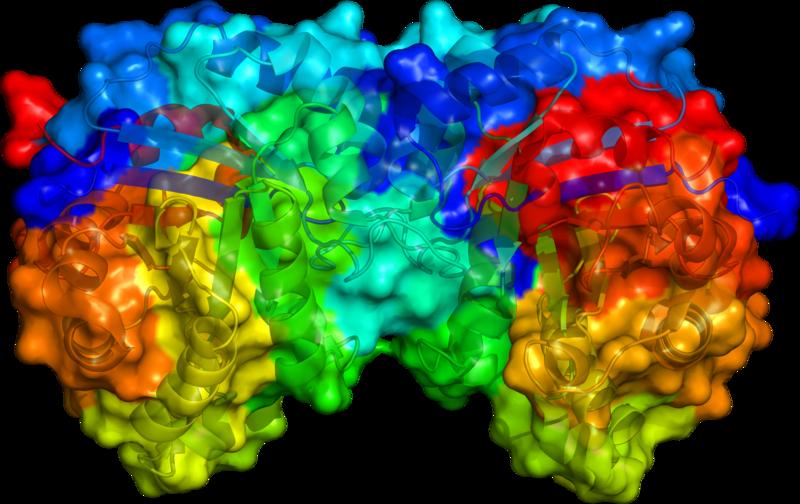
Structure
Triose phosphate isomerase is a dimer of identical subunits, each of which is made up of about 250 amino acid residues. The three-dimensional structure of a subunit contains eight α-helices (blue and red) on the outside and eight parallel β-strands on the inside (violet and yellow). This structural motif is called an αβ-barrel, or a TIM-barrel, and is by far the most commonly observed protein fold. The active site of this enzyme is in the center of the barrel. A glutamic acid residue is involved in the catalytic mechanism. The sequence around the active site residue is conserved in all known triose phosphate isomerases.
See also
References
http://pdbdev.sdsc.edu:48346/pdb/molecules/pdb50_6.html
ATP
ADP
ATP
ADP
+ +
NAD++ Pi
NADH + H+
NAD++ Pi
NADH + H+ H2O
H2O ADP
ATP
2 × Pyruvate 2 × File:Pyruvat.svg
|
de:Triosephosphatisomerase it:Trioso fosfato isomerasi Template:WH Template:WS