Tiocarlide
 | |
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
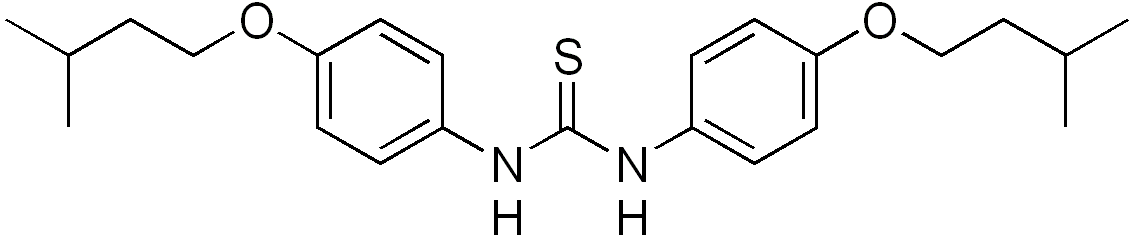
| Formula | C23H32N2O2S |
| Molar mass | 400.578 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Tiocarlide |
|
Articles |
|---|
|
Most recent articles on Tiocarlide |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Tiocarlide at Clinical Trials.gov Clinical Trials on Tiocarlide at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Tiocarlide
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Tiocarlide Discussion groups on Tiocarlide Patient Handouts on Tiocarlide Directions to Hospitals Treating Tiocarlide Risk calculators and risk factors for Tiocarlide
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Tiocarlide |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Thiocarlide (or tiocarlide or isoxyl) is a thiourea drug used in the treatment of tuberculosis, inhibiting synthesis of oleic acid and tuberculostearic acid.[1]
Thiocarlide has considerable antimycobacterial activity in vitro and is effective against multi-drug resistant strains of Mycobacterium tuberculosis.[2] Isoxyl inhibits M. bovis with six hours of exposure, which is similar to isoniazid and ethionamide, two other prominent anti-TB drugs. Unlike these two drugs, however, isoxyl also partially inhibits the synthesis of fatty acids.
Thiocarlide was developed by a Belgian company, Continental Pharma S.A. Belgo-Canadienne in Brussels, Belgium. The head researcher was Professor N. P. Buu-Hoi, head of Continental Pharma's Research Division.
References
- ↑ Phetsuksiri B, Jackson M, Scherman H; et al. (December 2003). "Unique mechanism of action of the thiourea drug isoxyl on Mycobacterium tuberculosis". J. Biol. Chem. 278 (52): 53123–30. doi:10.1074/jbc.M311209200. PMID 14559907.
- ↑ Phetsuksiri B, Baulard AR, Cooper AM; et al. (May 1999). "Antimycobacterial activities of isoxyl and new derivatives through the inhibition of mycolic acid synthesis". Antimicrob. Agents Chemother. 43 (5): 1042–51. PMC 89109. PMID 10223912.
- Pages with script errors
- CS1 maint: Explicit use of et al.
- CS1 maint: Multiple names: authors list
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Antibiotics
- Thioureas
- Phenol ethers
- Drug