Thiotepa
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Thiotepa is an antineoplastic agent that is FDA approved for the treatment of cns lymphoma, multiple myeloma, breast cancer, ovarian cancer, germ cell tumor, thalassemia, leukemia. Common adverse reactions include alopecia, injection site pain, rash, loss of appetite, nausea, vomiting, asthenia, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Haematological diseases
- The recommended dose in haematological diseases ranges from 125 mg/m2/day (3.38 mg/kg/day) to 300 mg/m2/day (8.10 mg/kg/day) as a single daily infusion, administered from 2 up to 4 consecutive days before autologous HPCT depending on the combination with other chemotherapeutic medicinal products, without exceeding the total maximum cumulative dose of 900 mg/m2 (24.32 mg/kg), during the time of the entire conditioning treatment.
LYMPHOMA
- The recommended dose ranges from 125 mg/m2/day (3.38 mg/kg/day) to 300 mg/m2/day (8.10 mg/ kg/day) as a single daily infusion, administered from 2 up to 4 consecutive days before autologous HPCT depending on the combination with other chemotherapeutic medicinal products, without exceeding the total maximum cumulative dose of 900 mg/m2 (24.32 mg/kg), during the time of the entire conditioning treatment.
CNS LYMPHOMA
- The recommended dose is 185 mg/m2/day (5 mg/kg/day) as a single daily infusion, administered for 2 consecutive days before autologous HPCT, without exceeding the total maximum cumulative dose of 370 mg/m2 (10 mg/kg), during the time of the entire conditioning treatment.
MULTIPLE MYELOMA
- The recommended dose ranges from 150 mg/m2/day (4.05 mg/kg/day) to 250 mg/m2/day (6.76 mg/ kg/day) as a single daily infusion, administered for 3 consecutive days before autologous HPCT depending on the combination with other chemotherapeutic medicinal products, without exceeding the total maximum cumulative dose of 750 mg/m2 (20.27 mg/kg), during the time of the entire conditioning treatment.
Solid tumours
- The recommended dose in solid tumours ranges from 120 mg/m2/day (3.24 mg/kg/day) to 250 mg/ m2/day (6.76 mg/kg/day) divided in one or two daily infusions, administered from 2 up to 5 consecutive days before autologous HPCT depending on the combination with other chemotherapeutic medicinal products, without exceeding the total maximum cumulative dose of 800 mg/m2 (21.62 mg/kg), during the time of the entire conditioning treatment.
BREAST CANCER
- The recommended dose ranges from 120 mg/m2/day (3.24 mg/kg/day) to 250 mg/m2/day (6.76 mg/ kg/day) as a single daily infusion, administered from 3 up to 5 consecutive days before autologous HPCT depending on the combination with other chemotherapeutic medicinal products, without exceeding the total maximum cumulative dose of 800 mg/m2 (21.62 mg/kg), during the time of the entire conditioning treatment.
CNS TUMOURS
- The recommended dose ranges from 125 mg/m2/day (3.38 mg/kg/day) to 250 mg/m2/day (6.76 mg/ kg/day) divided in one or two daily infusions, administered from 3 up to 4 consecutive days before autologous HPCT depending on the combination with other chemotherapeutic medicinal products, without exceeding the total maximum cumulative dose of 750 mg/m2 (20.27 mg/kg), during the time of the entire conditioning treatment.
OVARIAN CANCER
- The recommended dose is 250 mg/m2/day (6.76 mg/kg/day) as a single daily infusion, administered in 2 consecutive days before autologous HPCT, without exceeding the total maximum cumulative dose of 500 mg/m2 (13.51 mg/kg), during the time of the entire conditioning treatment.
GERM CELL TUMOURS
- The recommended dose ranges from 150 mg/m2/day (4.05 mg/kg/day) to 250 mg/m2/day (6.76 mg/ kg/day) as a single daily infusion, administered for 3 consecutive days before autologous HPCT depending on the combination with other chemotherapeutic medicinal products, without exceeding the total maximum cumulative dose of 750 mg/m2 (20.27 mg/kg), during the time of the entire conditioning treatment.
ALLOGENEIC HPCT
Haematological diseases
- The recommended dose in haematological diseases ranges from 185 mg/m2/day (5 mg/kg/day) to 481 mg/m2/day (13 mg/kg/day) divided in one or two daily infusions, administered from 1 up to 3 consecutive days before allogeneic HPCT depending on the combination with other chemotherapeutic medicinal products, without exceeding the total maximum cumulative dose of 555 mg/m2 (15 mg/kg), during the time of the entire conditioning treatment.
LYMPHOMA
- The recommended dose in lymphoma is 370 mg/m2/day (10 mg/kg/day) divided in two daily infusions before allogeneic HPCT, without exceeding the total maximum cumulative dose of 370 mg/m2 (10 mg/ kg), during the time of the entire conditioning treatment.
MULTIPLE MYELOMA
- The recommended dose is 185 mg/m2/day (5 mg/kg/day) as a single daily infusion before allogeneic HPCT, without exceeding the total maximum cumulative dose of 185 mg/m2 (5 mg/kg), during the time of the entire conditioning treatment.
LEUKEMIA
- The recommended dose ranges from 185 mg/m2/day (5 mg/kg/day) to 481 mg/m2/day (13 mg/kg/day) divided in one or two daily infusions, administered from 1 up to 2 consecutive days before allogeneic HPCT depending on the combination with other chemotherapeutic medicinal products, without exceeding the total maximum cumulative dose of 555 mg/m2 (15 mg/kg), during the time of the entire conditioning treatment.
THALASSEMIA
- The recommended dose is 370 mg/m2/day (10 mg/kg/day) divided in two daily infusions, administered before allogeneic HPCT, without exceeding the total maximum cumulative dose of 370 mg/m2 (10 mg/ kg), during the time of the entire conditioning treatment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Thiotepa in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Thiotepa in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
AUTOLOGOUS HPCT
Solid tumours
- The recommended dose in solid tumours ranges from 150 mg/m2/day (6 mg/kg/day) to 350 mg/ m2/day (14 mg/kg/day) as a single daily infusion, administered from 2 up to 3 consecutive days before autologous HPCT depending on the combination with other chemotherapeutic medicinal products, without exceeding the total maximum cumulative dose of 1050 mg/m2 (42 mg/kg), during the time of the entire conditioning treatment.
CNS TUMOURS
- The recommended dose ranges from 250 mg/m2/day (10 mg/kg/day) to 350 mg/m2/day (14 mg/kg/ day) as a single daily infusion, administered for 3 consecutive days before autologous HPCT depending on the combination with other chemotherapeutic medicinal products, without exceeding the total maximum cumulative dose of 1050 mg/m2 (42 mg/kg), during the time of the entire conditioning treatment.
ALLOGENEIC HPCT
Haematological diseases
- The recommended dose in haematological diseases ranges from 125 mg/m2/day (5 mg/kg/day) to 250 mg/ m2/day (10 mg/kg/day) divided in one or two daily infusions, administered from 1 up to 3 consecutive days before allogeneic HPCT depending on the combination with other chemotherapeutic medicinal products, without exceeding the total maximum cumulative dose of 375 mg/m2 (15 mg/kg), during the time of the entire conditioning treatment.
LEUKEMIA
- The recommended dose is 250 mg/m2/day (10 mg/kg/day) divided in two daily infusions, administered before allogeneic HPCT, without exceeding the total maximum cumulative dose of 250 mg/m2 (10 mg/ kg), during the time of the entire conditioning treatment.
THALASSEMIA
- The recommended dose ranges from 200 mg/m2/day (8 mg/kg/day) to 250 mg/m2/day (10 mg/kg/ day) divided in two daily infusions, administered before allogeneic HPCT without exceeding the total maximum cumulative dose of 250 mg/m2 (10 mg/kg), during the time of the entire conditioning treatment.
REFRACTORY CYTOPENIA
- The recommended dose is 125 mg/m2/day (5 mg/kg/day) as a single daily infusion, administered for 3 consecutive days before allogeneic HPCT, without exceeding the total maximum cumulative dose of 375 mg/m2 (15 mg/kg), during the time of the entire conditioning treatment.
GENETIC DISEASES
- The recommended dose is 125 mg/m2/day (5 mg/kg/day) as a single daily infusion, administered for 2 consecutive days before allogeneic HPCT, without exceeding the total maximum cumulative dose of 250 mg/m2 (10 mg/kg), during the time of the entire conditioning treatment.
SICKLE CELL ANAEMIA
- The recommended dose is 250 mg/m2/day (10 mg/kg/day) divided in two daily infusions, administered before allogeneic HPCT, without exceeding the total maximum cumulative dose of 250 mg/m2 (10 mg/ kg), during the time of the entire conditioning treatment.
Reconstitution
- TEPADINA® must be reconstituted with 1.5 ml of sterile water for injections.
- Using a syringe fitted with a needle, aseptically withdraw 1.5 ml of sterile water for injections.
- Inject the content of the syringe into the vial through the rubber stopper.
- Remove the syringe and the needle and mix manually by repeated inversions.
- Only clear colourless solutions, without any particulate matter, must be used.
Further dilution in the infusion bag
- The reconstituted solution is hypotonic and must be further diluted prior to administration with 500 ml sodium chloride 9 mg/ml (0.9 %) solution for injection (1000 ml if the dose is higher than 500 mg) or with an appropriate volume of sodium chloride 9 mg/ml (0.9 %) in order to obtain a final TEPADINA®concentration between 0.5 and 1 mg/ml.
Administration
- TEPADINA® infusion solution should be inspected visually for particulate matter and opalescence prior to administration. Solutions containing a precipitate should be discarded.
- It is recommended that the infusion solution be administered to patients using an infusion set equipped with a 0.2 µm in-line filter.
- TEPADINA® should be aseptically administered as a 2 - 4 hours infusion under room temperature and normal light conditions.
- Prior to and following each infusion, the indwelling catheter line should be flushed with approximately 5 ml sodium chloride 9 mg/ml (0.9 %) solution for injection.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Thiotepa in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Thiotepa in pediatric patients.
Contraindications
- Existing hepatic, renal or bone marrow damage (may use low dosage and frequent monitoring if need outweighs risk)
- Hypersensitivity to thiotepa
Warnings
There is limited information regarding Thiotepa Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Thiotepa Clinical Trials Experience in the drug label.
Postmarketing Experience
- Decrease in circulating blood cell counts (intended effect of the medicine to prepare you for your transplant infusion)
- Infection
- Liver disorders including blocking of a liver vein
- The graft attacks your body (graft versus host disease)
- Respiratory complications.
- Increased susceptibility to infection
- Whole-body inflammatory state (sepsis)
- Decreased counts of white blood cells, platelets and red blood cells (anaemia)
- The transplanted cells attack your body (graft versus host disease)
- Dizziness, headache, blurred vision
- Uncontrolled shaking of the body (convulsion)
- Sensation of tingling, pricking or numbness (paraesthesia)
- Partial loss of movement
- Cardiac arrest
- Nausea, vomiting, diarrhea
- Inflammation of the mucosa of the mouth (mucositis)
- Irritated stomach, gullet, intestine
- Inflammation of the liver
- High glucose in the blood
- Skin rash, itching, shedding
- Skin color disorder
- Redness of the skin (erythema)
- Hair loss
- Back and abdominal pain, pain
- Muscle and joint pain
- Abnormal electrical activity in the heart (arrhythmia)
- Inflammation of lung tissue
- Enlarged liver
- Altered organ function
- Blocking of a liver vein (VOD)
- Yellowing of the skin and eyes (jaundice)
- Hearing impaired
- Lymphatic obstruction
- High blood pressure
- Increased liver, renal and digestive enzymes
- Abnormal blood electrolytes
- Weight gain
- Fever, general weakness, chills
- Bleeding (haemorrhage)
- Nasal bleeding
- General swelling due to fluid retention (edema)
- Pain or inflammation at the injection site
- Eye infection (conjunctivitis)
- Decreased sperm cell count
- Vaginal bleeding
- Absence of menstrual periods (amenorrhea)
- Memory loss
- Delaying in weight and height increase
- Bladder disfunction
- Underproduction of testosterone
- Insufficient production of thyroid hormone
- Deficient activity of the pituitary gland
- Confusional state.
Drug Interactions
There is limited information regarding Thiotepa Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- You must tell your doctor if you are or think you may be pregnant before you receive TEPADINA® . You must not use TEPADINA® during pregnancy.
- Both women and men using TEPADINA® must use effective contraceptive methods during treatment.
- It is not known whether this medicinal product is excreted in breast milk. * As a precautionary measure, women must not breast-feed during treatment with TEPADINA® .
- TEPADINA® can impair male and female fertility. Male patients should seek for sperm preservation before therapy is started and should not father while treated and during the year after cessation of treatment.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Thiotepa in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Thiotepa during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Thiotepa with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Thiotepa with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Thiotepa with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Thiotepa with respect to specific gender populations.
Race
There is no FDA guidance on the use of Thiotepa with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Thiotepa in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Thiotepa in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Thiotepa in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Thiotepa in patients who are immunocompromised.
Others
(Description)
Administration and Monitoring
Administration
Monitoring
IV Compatibility
There is limited information regarding the compatibility of Thiotepa and IV administrations.
Overdosage
There is limited information regarding Thiotepa overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
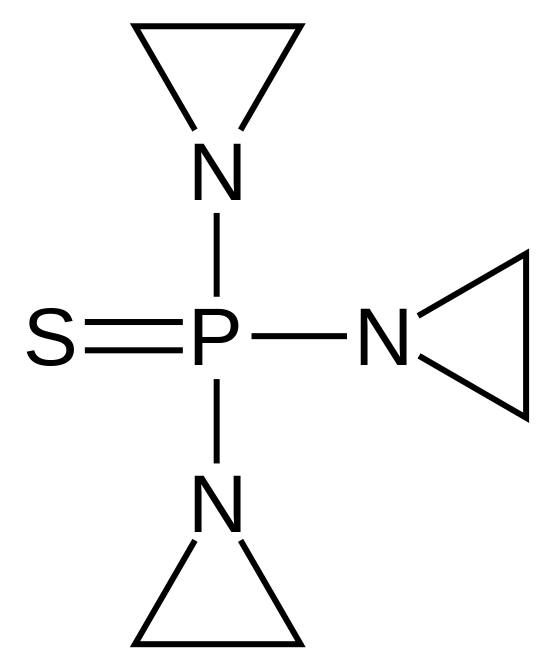
| |
Thiotepa
| |
| Systematic (IUPAC) name | |
| 1,1',1''-phosphorothioyltriaziridine | |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 189.23 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic (CYP2B, CYP3A) |
| Half life | 1.5-4.1 hours |
| Excretion | Renal 6 hours for ThioTEPA 8 hours for TEPA |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | IV, intracavitary, intravesical |
Mechanism of Action
- Thiotepa is a cytotoxic, ethylenimine-type agent related to nitrogen mustard, which acts by releasing ethylenimine radicals that disrupts DNA bonds and causes the breakage of links between the purine base and sugar, liberating alkylated guanines, resulting in the misreading of the DNA code and the inhibition of DNA, RNA, and protein synthesis in rapidly proliferating tumor cells.
Structure
(Description with picture)
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Thiotepa in the drug label.
Pharmacokinetics
Absorption
- Bioavailability, Oral: incomplete
- Distribution
- Vd: 0.3 to 1.6 L/kg
Metabolism
- Liver
- Metabolite, tepa: active
- Potent inhibitor of CYP2B6
Excretion
- Renal: less than 2% unchanged; 4.2% as active metabolite
- Total body clearance: 419 to 446 mL/min
- Dialyzable: Yes
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Thiotepa in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Thiotepa in the drug label.
How Supplied
There is limited information regarding Thiotepa How Supplied in the drug label.
Storage
- Keep out of the reach and sight of children.
- Do not use TEPADINA® after the expiry date which is stated on the carton and vial label, after EXP. The expiry date refers to the last day of that month.
- Store and transport refrigerated (2 °C - 8 °C).
- Do not freeze.
- After reconstitution the product is stable for 8 hours when stored at 2 °C - 8 °C.
- After dilution the product is stable for 24 hours when stored at 2 °C - 8 °C and for 4 hours when stored at 25 °C. From a microbiological point of view, the product should be used immediately.
- Any unused product or waste material should be disposed of in accordance with local requirements.
Images
Drug Images
{{#ask: Page Name::Thiotepa |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Thiotepa |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- WHAT TEPADINA® IS AND WHAT IT IS USED FOR
- TEPADINA® contains the active substance thiotepa, which belongs to a group of medicines called alkylating agents.
- TEPADINA® is used to prepare patients for bone marrow transplantation. It works by destroying bone marrow cells. This enables the transplantation of new bone marrow cells (haematopoietic progenitor cells), which in turn enable the body to produce healthy blood cells. TEPADINA® can be used in adults and children.
- BEFORE YOU USE TEPADINA®
- Do not use TEPADINA®
- If you are allergic (hypersensitive) to thiotepa,
- If you are pregnant or think you may be pregnant (see below),
- If you are breast-feeding,
- If you are receiving yellow fever vaccination, live virus and bacterial vaccines.
- Take special care with TEPADINA®
- You should tell your doctor if you have:
- You will have to take regular blood tests during treatment to check your blood cell counts.
- You will have to use anti-infectives to prevent and manage infections.
- TEPADINA® may cause another type of cancer in the future. Your doctor will discuss this risk with you.
Pregnancy and breast-feeding
- You must tell your doctor if you are or think you may be pregnant before you receive TEPADINA® . You must not use TEPADINA® during pregnancy.
- Both women and men using TEPADINA® must use effective contraceptive methods during treatment.
- It is not known whether this medicinal product is excreted in breast milk. * As a precautionary measure, women must not breast-feed during treatment with TEPADINA® .
- TEPADINA® can impair male and female fertility. Male patients should seek for sperm preservation before therapy is started and should not father while treated and during the year after cessation of treatment.
Using other medicines
- Please tell your doctor if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
- HOW TO USE TEPADINA®
- Your doctor will calculate the dose according to your body surface or weight and your disease.
- How TEPADINA® is given
- TEPADINA® is administered by a qualified healthcare professional as an intravenous infusion (drip in a vein) after dilution of the individual vial. * Each infusion will last 2 - 4 hours.
Frequency of administration
- You will receive your infusions every 12 or 24 hours. The duration of treatment can last up to 3 days. Frequency of administration and duration of treatment depend on your disease.
- POSSIBLE SIDE EFFECTS
- Like all medicines, TEPADINA® can cause side effects, although not everybody gets them.
- The most serious side effects of TEPADINA® therapy or the transplant procedure may include
- Decrease in circulating blood cell counts (intended effect of the medicine to prepare you for your transplant infusion)
- Infection
- Liver disorders including blocking of a liver vein
- The graft attacks your body (graft versus host disease)
- Respiratory complications
- Your doctor will monitor your blood counts and liver enzymes regularly to detect and manage these events.
- Side effects of TEPADINA® may occur with certain frequencies, which are defined as follows:
- Very common
- Affects more than 1 user in 10
- Common
- Affects 1 to 10 users in 100
- Uncommon
- Affects 1 to 10 users in 1,000
- Rare
- Affects 1 to 10 users in 10,000
- Very rare
- Affects less than 1 user in 10,000
- Not known
- Frequency cannot be estimated from the available data.
Very common side effects
- Increased susceptibility to infection
- Whole-body inflammatory state (sepsis)
- Decreased counts of white blood cells, platelets and red blood cells (anaemia)
- The transplanted cells attack your body (graft versus host disease)
- Dizziness, headache, blurred vision
- Uncontrolled shaking of the body (convulsion)
- Sensation of tingling, pricking or numbness (paraesthesia)
- Partial loss of movement
- Cardiac arrest
- Nausea, vomiting, diarrhoea
- Inflammation of the mucosa of the mouth (mucositis)
- Irritated stomach, gullet, intestine
- Inflammation of the colon
- Anorexia, decreased appetite
- High glucose in the blood
- Skin rash, itching, shedding
- Skin colour disorder (do not confuse with jaundice - see below)
- Redness of the skin (erythema)
- Hair loss
- Back and abdominal pain, pain
- Muscle and joint pain
- Abnormal electrical activity in the heart (arrhythmia)
- Inflammation of lung tissue
- Enlarged liver
- Altered organ function
- Blocking of a liver vein (VOD)
- Yellowing of the skin and eyes (jaundice)
- Hearing impaired
- Lymphatic obstruction
- High blood pressure
- Increased liver, renal and digestive enzymes
- Abnormal blood electrolytes
- Weight gain
- Fever, general weakness, chills
- Bleeding (haemorrhage)
- Nasal bleeding
- General swelling due to fluid retention (oedema)
- Pain or inflammation at the injection site
- Eye infection (conjunctivitis)
- Decreased sperm cell count
- Vaginal bleeding
- Absence of menstrual periods (amenorrhea)
- Memory loss
- Delaying in weight and height increase
- Bladder disfunction
- Underproduction of testosterone
- Insufficient production of thyroid hormone
- Deficient activity of the pituitary gland
- Confusional state
Common side effects
- Anxiety, confusion
- Abnormal bulging outward of one of the arteries in the brain (intracranial aneurysm)
- Creatinine elevated
- Allergic reactions
- Occlusion of a blood vessel (embolism)
- Heart rhythm disorder
- Heart inability
- Cardiovascular inability
- Oxygen deficiency
- Fluid accumulation in the lungs (pulmonary oedema)
- Pulmonary bleeding
- Respiratory arrest
- Blood in the urine (haematuria) and moderate renal insufficiency
- Inflammation of the urinary bladder
- Discomfort in urination and decrease in urine output (disuria and oliguria)
- Increase in the amount of nitrogen components in the blood stream (BUN increase)
- Cataract
- Inability of the liver
- Cerebral haemorrhage
- Cough
- Constipation and upset stomach
- Obstruction of the bowel
- Perforation of stomach
- Changes in muscle tone
- Gross lack of coordination of muscle movements
- Bruises due to a low platelet count
- Menopausal symptoms
- Cancer (second primary malignancies)
- Abnormal brain function
Uncommon side effects
- Inflammation and exfoliation of the skin (erythrodermic psoriasis), delirium, nervousness, hallucination, agitation, gastrointestinal ulcer
- Inflammation of the muscular tissue of the heart (myocarditis), abnormal heart condition (cardiomyopathy)
- Male and female infertility
- If any of the side effects gets serious, or if you notice any side effects not mentioned in this leaflet, please tell your doctor or nurse.
FURTHER INFORMATION
- What TEPADINA® contains
- The active substance is thiotepa. One vial contains 15 mg thiotepa. After reconstitution, each ml contains 10 mg thiotepa (10 mg/ml).
- TEPADINA ® does not contain any other ingredients.
- What TEPADINA® looks like and contents of the pack
- TEPADINA® is a white crystalline powder supplied in a glass vial containing 15 mg thiotepa.
- Each carton contains 1 vial.
Precautions with Alcohol
Alcohol-Thiotepa interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- TEPADINA ®[1]
Look-Alike Drug Names
There is limited information regarding Thiotepa Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.