Desflurane
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Desflurane is a general anesthetic that is FDA approved for the {{{indicationType}}} of general anesthesia. Common adverse reactions include gastrointestinal: nausea (27% ), vomiting (16% ), respiratory: cough ( adult induction, 22% to 34% ), interrupted breathing (adult induction, 30% to 39%; adult and intubated pediatric maintenance, greater than 1% ).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- General anesthesia: induction, initial inhaled concentration of 3% in oxygen or nitrous oxide/oxygen, increased by 0.5% to 1% increments every 2 to 3 breaths or as tolerated (end tidal concentrations 4% to 11%) inspired concentrations greater than 12% have been safely administered during induction and may require a reduction of nitrous oxide or air.
- General anesthesia: maintenance, inhaled concentrations of 2.5% to 8.5% with or without concomitant nitrous oxide; dosage must be individualized based on patient response.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Desflurane in adult patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Desflurane in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- General anesthesia: maintenance, inhaled in concentrations of 5.2% to 10% with or without concomitant nitrous oxide; dosage must be individualized based on patient response
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Desflurane in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Desflurane in pediatric patients.
Contraindications
- The use of desflurane (desflurane, USP) is contraindicated in the following conditions:
- Known or suspected genetic susceptibility to malignant hyperthermia.
- Patients in whom general anesthesia is contraindicated.
- Induction of anesthesia in pediatric patients.
- Patients with known sensitivity to desflurane (desflurane, USP) or to other halogenated agents [see Warnings and Precautions (5.5)].
- Patients with a history of confirmed hepatitis or with a history of unexplained moderate to severe hepatic dysfunction (e.g., jaundice associated with fever and/or eosinophilia) after anesthesia with desflurane (desflurane, USP) or other halogenated agents [see Warnings and Precautions].
Warnings
Malignant Hyperthermia
- In susceptible individuals, potent inhalation anesthetic agents may trigger a skeletal muscle hypermetabolic state leading to high oxygen demand and the clinical syndrome known as malignant hyperthermia. In genetically susceptible pigs, desflurane induced malignant hyperthermia. The clinical syndrome is signaled by hypercapnia, and may include muscle rigidity, tachycardia, tachypnea, cyanosis, arrhythmias, and/or unstable blood pressure. Some of these nonspecific signs may also appear during light anesthesia: acute hypoxia, hypercapnia, and hypovolemia.
- Treatment of malignant hyperthermia includes discontinuation of triggering agents, administration of intravenous dantrolene sodium, and application of supportive therapy. (Consult prescribing information for dantrolene sodium intravenous for additional information on patient management.) Renal failure may appear later, and urine flow should be monitored and sustained if possible.
- Fatal outcome of malignant hyperthermia has been reported with desflurane.
Perioperative Hyperkalemia
- Use of inhaled anesthetic agents has been associated with rare increases in serum potassium levels that have resulted in cardiac arrhythmias and death in pediatric patients during the postoperative period. Patients with latent as well as overt neuromuscular disease, particularly Duchenne muscular dystrophy, appear to be most vulnerable. Concomitant use of succinylcholine has been associated with most, but not all, of these cases. These patients also experienced significant elevations in serum creatinine kinase levels and, in some cases, changes in urine consistent with myoglobinuria. Despite the similarity in presentation to malignant hyperthermia, none of these patients exhibited signs or symptoms of muscle rigidity or hypermetabolic state. Early and aggressive intervention to treat the hyperkalemia and resistant arrhythmias is recommended, as is subsequent evaluation for latent neuromuscular disease.
Respiratory Adverse Reactions in Pediatric Patients
- Due to the limited data available in non-intubated pediatric patients, Desflurane (desflurane, USP) is not approved for maintenance of anesthesia in non-intubated children due to an increased incidence of respiratory adverse reactions, including coughing, laryngospasm and secretions [see Clinical Studies ].
- Caution should be exercised should Desflurane (desflurane, USP) be used for maintenance of anesthesia with laryngeal mask airway (LMA™ mask) in children, in particular for children 6 years old or younger because of the increased potential for adverse respiratory reactions, e.g. coughing and laryngospasm, especially with removal of the LMA™ mask under deep anesthesia [see Clinical Studies].
- Caution should be exercised when Desflurane (desflurane, USP) is used for maintenance of anesthesia in children with asthma or a history of recent upper airway infection due to the potential for airway narrowing and increases in airway resistance.
Interactions with Desiccated Carbon Dioxide Absorbents
- Desflurane like some other inhalation anesthetics, can react with desiccated carbon dioxide (CO2) absorbents to produce carbon monoxide that may result in elevated levels of carboxyhemoglobin in some patients. Case reports suggest that barium hydroxide lime and soda lime become desiccated when fresh gases are passed through the CO2 canister at high flow rates over many hours or days. When a clinician suspects that CO2 absorbent may be desiccated, it should be replaced before the administration of desflurane (desflurane, USP).
Hepatobiliary Disorders
- With the use of halogenated anesthetics, disruption of hepatic function, icterus and fatal liver necrosis have been reported; such reactions appear to indicate hypersensitivity. As with other halogenated anesthetic agents, desflurane (desflurane, USP) may cause sensitivity hepatitis in patients who have been sensitized by previous exposure to halogenated anesthetics [see Contraindications]. Cirrhosis, viral hepatitis or other pre-existing hepatic disease may be a reason to select an anesthetic other than a halogenated anesthetic. As with all halogenated anesthetics, repeated anesthesia within a short period of time should be approached with caution.
Laboratory Findings
- Transient elevations in glucose and white blood cell count may occur as with use of other anesthetic agents.
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience
- Adverse event information is derived from controlled clinical trials, the majority of which were conducted in the United States. The studies were conducted using a variety of premedications, other anesthetics, and surgical procedures of varying length. Most adverse events reported were mild and transient, and may reflect the surgical procedures, patient characteristics (including disease) and/or medications administered.
- Of the 2,143 patients exposed to Desflurane (desflurane, USP) in clinical trials, 370 adults and 152 children were induced with desflurane alone and 987 patients were maintained principally with desflurane. The frequencies given reflect the percent of patients with the event. Each patient was counted once for each type of adverse event. They are presented in alphabetical order according to body system.

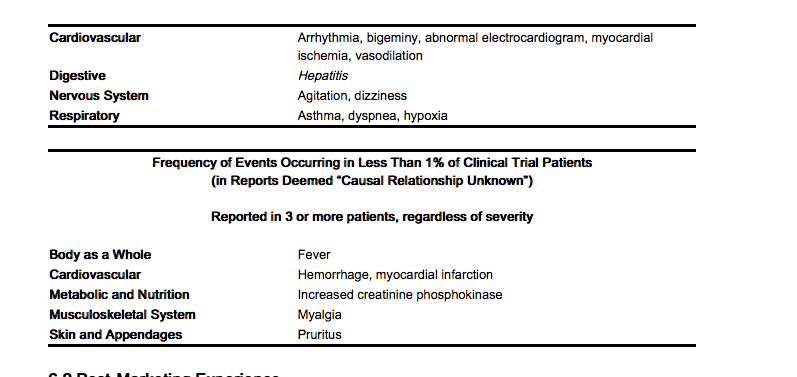
- Frequency of Events Occurring in Less Than 1% of Patients
(in Reports Deemed “Probably Causally Related”)
- Reported in 3 or more patients, regardless of severity
- Adverse reactions reported only from postmarketing experience or in the literature, not seen in clinical trials, are considered rare and are italicized.
Postmarketing Experience
Post-Marketing Experience
- The following adverse reactions have been identified during post-approval use of desflurane (desflurane, USP). Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders
Metabolism and Nutrition Disorders
Nervous System Disorders
Eye Disorders
- Ocular icterus
Cardiac Disorders
- Cardiac arrest, Torsade de pointes, ventricular failure, ventricular hypokinesia, Atrial fibrillation
Vascular Disorders
Respiratory, Thoracic and Mediastinal Disorders
Gastrointestinal Disorders
- Pancreatitis acute, abdominal pain
Hepatobiliary Disorders
- Hepatic failure, hepatic necrosis, Hepatitis, cytolytic hepatitis, cholestasis, jaundice, hepatic function abnormal, liver disorder
Skin and Subcutaneous Tissue Disorder
Musculoskeletal, Connective Tissue and Bone Disorders
General Disorders and Administration Site Conditions
- Hyperthermia malignant, asthenia, malaise
Investigations
- Electrocardiogram ST-T change, electrocardiogram T-wave inversion, tranaminases increased, alanine aminotransferase increased, aspartate aminotransferase increased, Blood bilirubin increased, coagulation test abnormal, ammonia increased
Injury, Poisoning, and Procedural Complications
- Tachyarrhythmia, palpitations, eye burns, blindness transient, encephalopathy, ulcerative keratitis, ocular hyperemia, visual acuity reduced, eye irritation, eye pain, dizziness, migraine, fatigue, accidental exposure, skin burning sensation, drug administration error
- All of reactions categorized within this SOC were accidental exposures to non-patients.
Drug Interactions
- No clinically significant adverse interactions with commonly used preanesthetic drugs, or drugs used during anesthesia (muscle relaxants, intravenous agents, and local anesthetic agents) were reported in clinical trials. The effect of desflurane (desflurane, USP) on the disposition of other drugs has not been determined. Similar to isoflurane, desflurane (desflurane, USP) does not predispose to premature ventricular arrhythmias in the presence of exogenously infused epinephrine in swine.
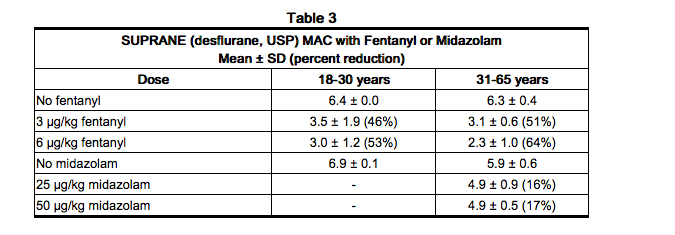
Benzodiazepines and Opioids (MAC Reduction)
- Benzodiazepines and opioids decrease the amount of desflurane (MAC) needed to produce anesthesia. This effect is shown in Table 3 for intravenous midazolam (25-50 µg/kg) and intravenous fentanyl (3-6 µg/kg) in patients of two different age groups
Neuromuscular Blocking Agents
- Anesthetic concentrations of desflurane at equilibrium (administered for 15 or more minutes before testing) reduced the ED95 of succinylcholine by approximately 30% and that of atracurium and pancuronium by approximately 50% compared to N2O/opioid anesthesia (see Table 4). The effect of desflurane on duration of nondepolarizing neuromuscular blockade has not been studied.
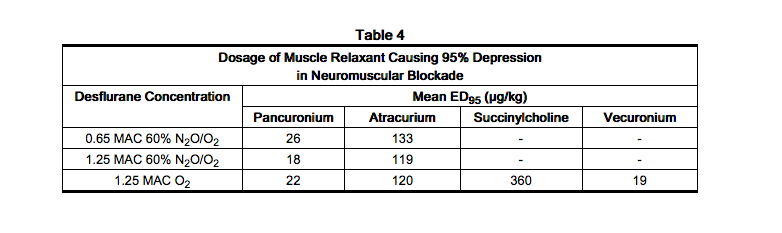
- Dosage reduction of neuromuscular blocking agents during induction of anesthesia may result in delayed onset of conditions suitable for endotracheal intubation or inadequate muscle relaxation, because potentiation of neuromuscular blocking agents requires equilibration of muscle with the delivered partial pressure of desflurane (desflurane, USP).
- Among nondepolarizing drugs, pancuronium, atracurium, and vecuronium interactions have been studied. In the absence of specific guidelines:
- For endotracheal intubation, do not reduce the dose of nondepolarizing muscle relaxants or succinylcholine.
- During maintenance of anesthesia, the dose of nondepolarizing muscle relaxants is likely to be reduced compared to that during N2O/opioid anesthesia. Administration of supplemental doses of muscle relaxants should be guided by the response to nerve stimulation.
Concomitant use with N2O
- Concomitant administration of N2O reduces the MAC of desflurane (desflurane, USP) [see Dosage and Administration (2), Table 1].
Beta Blockers
- Concomitant use of beta blockers may exaggerate the cardiovascular effects of inhalational anesthetics, including hypotension and negative inotropic effects.
Monoamine Oxidase Inhibitors (MAO)
- Concomitant use of MAO inhibitors and inhalational anesthetics may increase the risk of hemodynamic instability during surgery or medical procedures.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. Reproduction studies have been performed in rats at doses up to at 1 MAC hour for a minimum of 21 days and have revealed no evidence of impaired fertility or harm to the fetus due to desflurane (desflurane, USP).
- No teratogenic effect was observed at approximately 10 and 13 cumulative MAC-Hour exposures at 1 MAC-Hour per day during organogenesis in rats or rabbits. At higher doses increased incidences of post-implantation loss and maternal toxicity were observed. However, at 10 MAC-Hours cumulative exposure in rats, about 6% decrease in the weight of male pups was observed at preterm caesarean delivery.
- Rats exposed to desflurane (desflurane, USP) at 1 MAC-Hour per day from gestation day 15 to lactation day 21, did not show signs of dystocia. * Body weights of pups delivered by these dams at birth and during lactation were comparable to that of control pups. No treatment related behavioral changes were reported in these pups during lactation.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Desflurane in women who are pregnant.
Labor and Delivery
- The safety of desflurane (desflurane, USP) during labor or delivery has not been demonstrated. desflurane (desflurane, USP) is a uterine-relaxant.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when desflurane (desflurane, USP) is administered to a nursing woman.
Pediatric Use
- Respiratory Adverse Reactions in Pediatric Patients
- Desflurane (desflurane, USP) is indicated for maintenance of anesthesia in infants and children after induction of anesthesia with agents other than desflurane (desflurane, USP), and tracheal intubation.
- Due to limited data available in non-intubated pediatric patients, desflurane (desflurane, USP) is not approved for maintenance of anesthesia in non-intubated children due to an increased incidence of respiratory adverse reactions, including coughing (26%), laryngospasm (13%) and secretions (12%) [see Clinical Studies].
- Caution should be exercised should desflurane (desflurane, USP) be used for maintenance anesthesia with laryngeal mask airway (LMA™ mask) in children, in particular for children 6 years old or younger because of the increased potential for adverse respiratory reactions, e.g. coughing and laryngospasm, especially with removal of the LMA™ mask under deep anesthesia [see Clinical Studies].
- Caution should be exercised when Desflurane (desflurane, USP) is used for maintenance of anesthesia in children with asthma or a history of recent upper airway infection due to the potential for airway narrowing and increases in airway resistance.
Geriatic Use
- The minimum alveolar concentration (MAC) of desflurane (desflurane, USP) decreases with increasing patient age. The dose should be adjusted accordingly. The average MAC for Desflurane (desflurane, USP) in a 70 year old patient is two-thirds the MAC for a 20 year old patient [see Dosage and Administration Table 1 and Clinical Studies].
Gender
There is no FDA guidance on the use of Desflurane with respect to specific gender populations.
Race
There is no FDA guidance on the use of Desflurane with respect to specific racial populations.
Renal Impairment
- Concentrations of 1-4% desflurane (desflurane, USP) in nitrous oxide/oxygen have been used in patients with chronic renal or hepatic impairment and during renal transplantation surgery.
- Because of minimal metabolism, a need for dose adjustment in patients with renal and hepatic impairment is not to be expected.
- Nine patients receiving desflurane (N=9) were compared to 9 patients receiving isoflurane, all with chronic renal insufficiency (serum creatinine 1.5-6.9 mg/dL). No differences in hematological or biochemical tests, including renal function evaluation, were seen between the two groups. Similarly, no differences were found in a comparison of patients receiving either desflurane (N=28) or isoflurane (N=30) undergoing renal transplant.
Hepatic Impairment
- Eight patients receiving desflurane (desflurane, USP) were compared to six patients receiving isoflurane, all with chronic hepatic disease (viral hepatitis, alcoholic hepatitis, or cirrhosis). No differences in hematological or biochemical tests, including hepatic enzymes and hepatic function evaluation, were seen.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Desflurane in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Desflurane in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Desflurane Administration in the drug label.
Monitoring
There is limited information regarding Desflurane Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Desflurane and IV administrations.
Overdosage
- The symptoms of overdosage of desflurane (desflurane, USP) can present as a deepening of anesthesia, cardiac and/or respiratory depression in spontaneously breathing patients, and cardiac depression in ventilated patients in whom hypercapnia and hypoxia may occur only at a late stage. In the event of overdosage, or suspected overdosage, take the following actions: discontinue administration of desflurane (desflurane, USP), maintain a patent airway, initiate assisted or controlled ventilation with oxygen, and maintain adequate cardiovascular function.
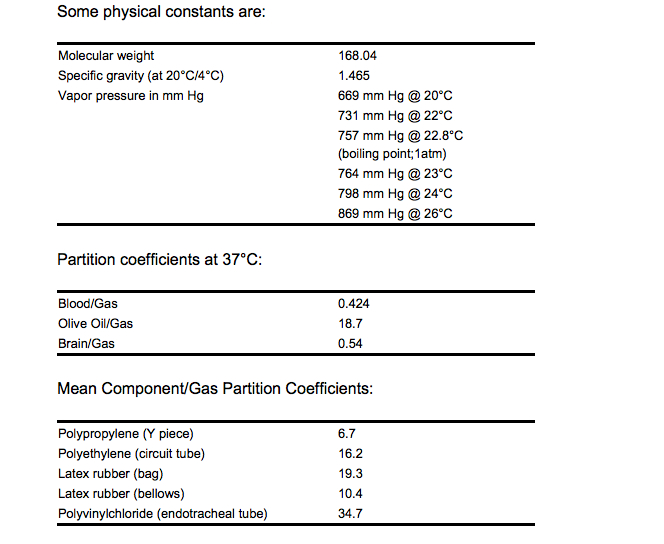
Pharmacology
| |
| |
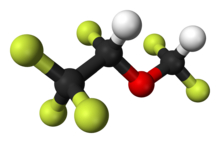
Desflurane
| |
| Systematic (IUPAC) name | |
| 2-(difluoromethoxy)-1,1,1,2-tetrafluoro-ethane | |
| Identifiers | |
| CAS number | |
| ATC code | N01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 168.038 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Not metabolized |
| Half life | Elimination dependent on minute ventilation |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
There is limited information regarding Desflurane Mechanism of Action in the drug label.
Structure
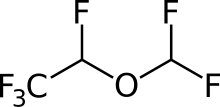
- Desflurane (desflurane, USP), a nonflammable liquid administered via vaporizer, is a general inhalation anesthetic. It is (±)1,2,2,2-tetrafluoroethyl difluoromethyl ether:
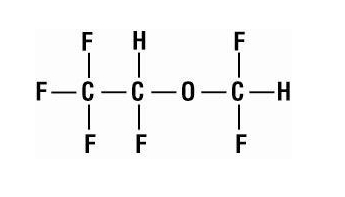
- Desflurane (desflurane, USP) is nonflammable as defined by the requirements of International Electrotechnical Commission 601-2-13.
- Desflurane (desflurane, USP) is a colorless, volatile liquid below 22.8°C. Data indicate that Desflurane (desflurane, USP) is stable when stored under normal room lighting conditions according to instructions.
- Desflurane (desflurane, USP) is chemically stable. The only known degradation reaction is through prolonged direct contact with soda lime producing low levels of fluoroform (CHF3). The amount of CHF3 obtained is similar to that produced with MAC-equivalent doses of isoflurane. No discernible degradation occurs in the presence of strong acids.
- Desflurane (desflurane, USP) does not corrode stainless steel, brass, aluminum, anodized aluminum, nickel plated brass, copper, or beryllium
Pharmacodynamics
- Changes in the clinical effects of Desflurane (desflurane, USP) rapidly follow changes in the inspired concentration. The duration of anesthesia and selected recovery measures for Desflurane (desflurane, USP) are given in the following tables:
- In 178 female outpatients undergoing laparoscopy, premedicated with fentanyl (1.5-2.0 µg/kg), anesthesia was initiated with propofol 2.5 mg/kg, desflurane/N2O 60% in O2 or desflurane/O2 alone. Anesthesia was maintained with either propofol 1.5-9.0 mg/kg/hr, desflurane 2.6-8.4% in N2O 60% in O2, or desflurane 3.1-8.9% in O2.
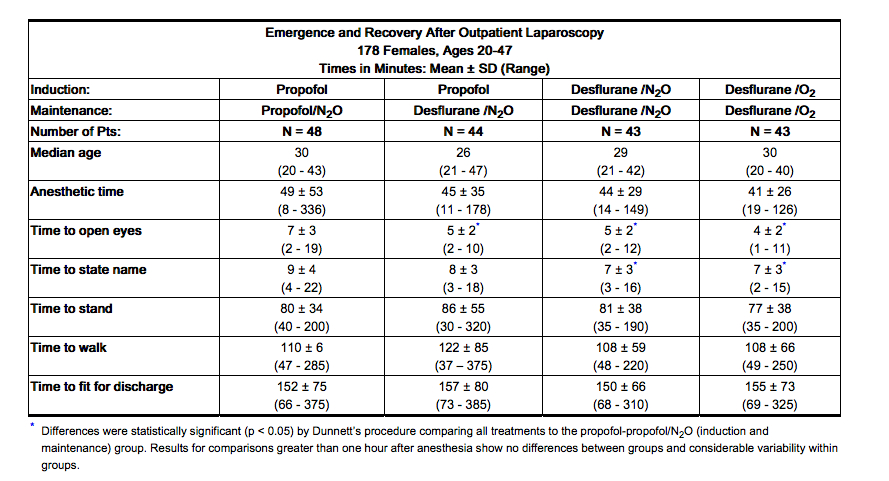
- In 88 unpremedicated outpatients, anesthesia was initiated with thiopental 3-9 mg/kg or desflurane in O2. Anesthesia was maintained with isoflurane 0.7-1.4% in N2O 60%, desflurane 1.8-7.7% in N2O 60%, or desflurane 4.4-11.9% in O2.
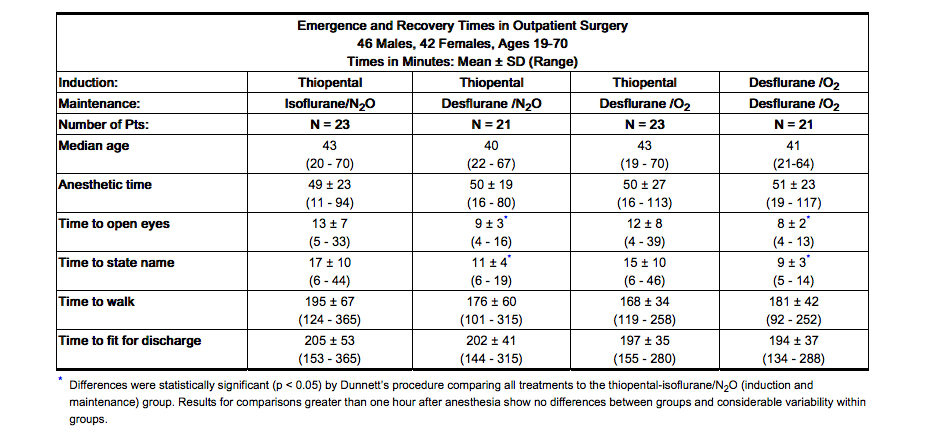
- Recovery from anesthesia was assessed at 30, 60, and 90 minutes following 0.5 MAC desflurane (3%) or isoflurane (0.6%) in N2O 60% using subjective and objective tests. At 30 minutes after anesthesia, only 43% of patients in the isoflurane group were able to perform the psychometric tests compared to 76% in the Desflurane (desflurane, USP) group (p < 0.05).
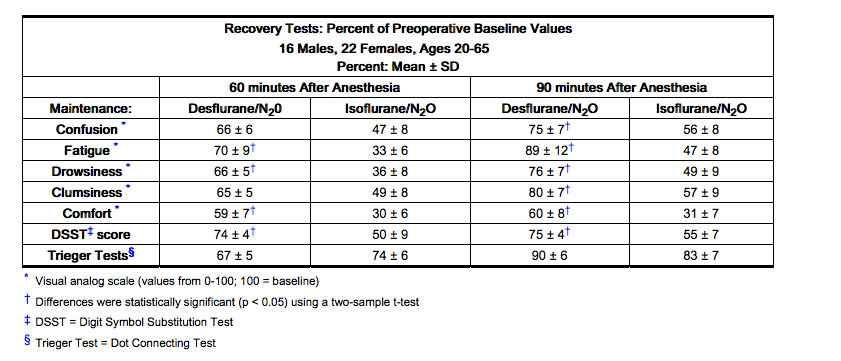
- Desflurane (desflurane, USP) was studied in twelve volunteers receiving no other drugs. Hemodynamic effects during controlled ventilation (PaCO2 38 mm Hg) were:

- When the same volunteers breathed spontaneously during desflurane anesthesia, systemic vascular resistance and mean arterial blood pressure decreased; cardiac index, heart rate, stroke volume, and central venous pressure (CVP) increased compared to values when the volunteers were conscious. Cardiac index, stroke volume, and CVP were greater during spontaneous ventilation than during controlled ventilation.
- During spontaneous ventilation in the same volunteers, increasing the concentration of Desflurane (desflurane, USP) from 3% to 12% decreased tidal volume and increased arterial carbon dioxide tension and respiratory rate. The combination of N2O 60% with a given concentration of desflurane gave results similar to those with desflurane alone. Respiratory depression produced by desflurane is similar to that produced by other potent inhalation agents.
- The use of desflurane concentrations higher than 1.5 MAC may produce apnea.
Pharmacokinetics
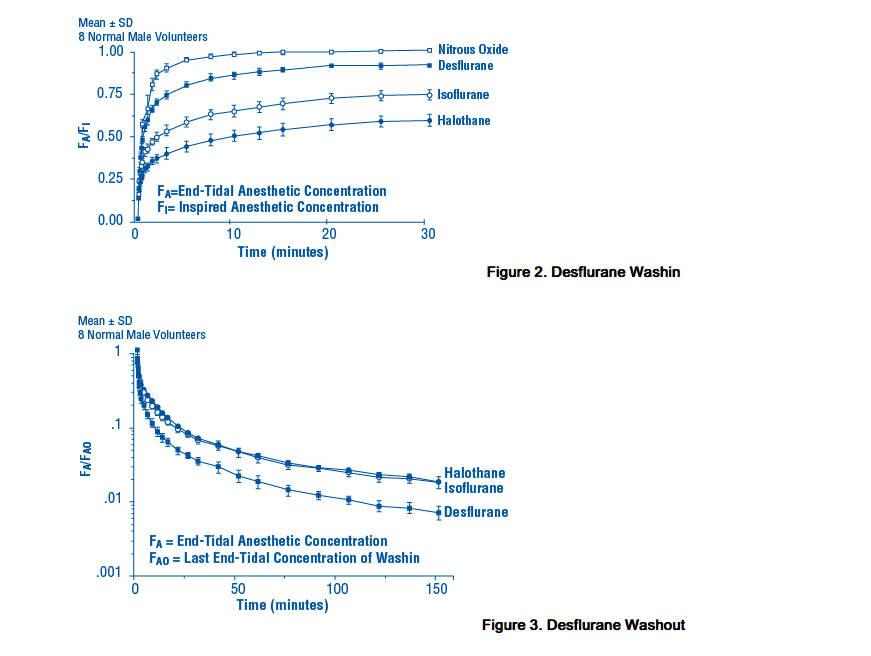
- Due to the volatile nature of desflurane in plasma samples, the washin-washout profile of desflurane was used as a surrogate of plasma pharmacokinetics. Desflurane (desflurane, USP) is a volatile liquid inhalation anesthetic minimally biotransformed in the liver in humans. Less than 0.02% of the desflurane absorbed can be recovered as urinary metabolites (compared to 0.2% for isoflurane). Eight healthy male volunteers first breathed 70% N2O/30% O2 for 30 minutes and then a mixture of desflurane 2.0%, isoflurane 0.4%, and halothane 0.2% for another 30 minutes. During this time, inspired and end-tidal concentrations (FI and FA) were measured. The FA/FI (washin) value at 30 minutes for desflurane was 0.91, compared to 1.00 for N2O, 0.74 for isoflurane, and 0.58 for halothane (see Figure 2). The washin rates for halothane and isoflurane were similar to literature values. The washin was faster for desflurane than for isoflurane and halothane at all time points. The FA/FAO (washout) value at 5 minutes was 0.12 for desflurane, 0.22 for isoflurane, and 0.25 for halothane (see Figure 3). The washout for desflurane was more rapid than that for isoflurane and halothane at all elimination time points. By 5 days, the FA/FAO for desflurane is 1/20th of that for halothane or isoflurane.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Animal carcinogenicity studies have not been performed with Desflurane (desflurane, USP). In vitro and in vivo genotoxicity studies did not demonstrate mutagenicity or chromosomal damage by Desflurane (desflurane, USP). Tests for genotoxicity included the Ames mutation assay, the metaphase analysis of human lymphocytes, and the mouse micronucleus assay.
- Fertility was not affected after 1 MAC-Hour per day exposure (cumulative 63 and 14 MAC-Hours for males and females, respectively). At higher doses, parental toxicity (mortalities and reduced weight gain) was observed which could affect fertility.
Clinical Studies
- The efficacy of Desflurane (desflurane, USP) was evaluated in 1,843 patients including ambulatory (N=1,061), cardiovascular (N=277), geriatric (N=103), neurosurgical (N=40), and pediatric (N=235) patients. Clinical experience with these patients and with 1,087 control patients in these studies not receiving Desflurane (desflurane, USP) is described below. Although Desflurane (desflurane, USP) can be used in adults for the inhalation induction of anesthesia via mask, it produces a high incidence of respiratory irritation (coughing, breathholding, apnea, increased secretions, laryngospasm). Oxyhemoglobin saturation below 90% occurred in 6% of patients (from pooled data, N = 370 adults).
Ambulatory Surgery
- Desflurane (desflurane, USP) plus N2O was compared to isoflurane plus N2O in multicenter studies (21 sites) of 792 ASA physical status I, II, or III patients aged 18-76 years (median 32).
Induction
- Anesthetic induction begun with thiopental and continued with Desflurane (desflurane, USP) was associated with a 7% incidence of oxyhemoglobin saturation of 90% or less (from pooled data, N = 307) compared with 5% in patients in whom anesthesia was induced with thiopental and isoflurane (from pooled data, N = 152).
Maintenance & Recovery
- Desflurane (desflurane, USP) with or without N2O or other anesthetics was generally well tolerated. There were no differences between Desflurane (desflurane, USP) and the other anesthetics studied in the times that patients were judged fit for discharge.
- In one outpatient study, patients received a standardized anesthetic consisting of thiopental 4.2-4.4 mg/kg, fentanyl 3.5-4.0 µg/kg, vecuronium 0.05-0.07 mg/kg, and N2O 60% in oxygen with either desflurane 3% or isoflurane 0.6%. Emergence times were significantly different; but times to sit up and discharge were not different (see Table 5).

Cardiovascular Surgery
- Desflurane (desflurane, USP) was compared to isoflurane, sufentanil or fentanyl for the anesthetic management of coronary artery bypass graft (CABG), abdominal aortic aneurysm, peripheral vascular and carotid endarterectomy surgery in 7 studies at 15 centers involving a total of 558 patients. In all patients except the desflurane vs. sufentanil study, the volatile anesthetics were supplemented with intravenous opioids, usually fentanyl. Blood pressure and heart rate were controlled by changes in concentration of the volatile anesthetics or opioids and cardiovascular drugs if necessary. Oxygen (100%) was the carrier gas in 253 of 277 desflurane cases (24 of 277 received N2O/O2).

- No differences were found in cardiovascular outcome (death, myocardial infarction, ventricular tachycardia or fibrillation, heart failure) among desflurane and the other anesthetics.
Induction
- Desflurane (desflurane, USP) should not be used as the sole agent for anesthetic induction in patients with coronary artery disease or any patients where increases in heart rate or blood pressure are undesirable. In the desflurane vs. sufentanil study, anesthetic induction with desflurane without opioids was associated with new transient ischemia in 14 patients vs. 0 in the sufentanil group. In the desflurane group, mean heart rate, arterial pressure, and pulmonary blood pressure increased and stroke volume decreased in contrast to no change in the sufentanil group. Cardiovascular drugs were used frequently in both groups: especially esmolol in the desflurane group (56% vs. 0%) and phenylephrine in the sufentanil group (43% vs. 27%). When 10 µg/kg of fentanyl was used to supplement induction of anesthesia at one other center, continuous 2-lead ECG analysis showed a low incidence of myocardial ischemia and no difference between desflurane and isoflurane. If desflurane is to be used in patients with coronary artery disease, it should be used in combination with other medications for induction of anesthesia, preferably intravenous opioids and hypnotics.
Maintenance & Recovery
- In studies where Desflurane (desflurane, USP) or isoflurane anesthesia was supplemented with fentanyl, there were no differences in hemodynamic variables or the incidence of myocardial ischemia in the patients anesthetized with desflurane compared to those anesthetized with isoflurane.
- During the precardiopulmonary bypass period, in the desflurane vs. sufentanil study where the desflurane patients received no intravenous opioid, more desflurane patients required cardiovascular adjuvants to control hemodynamics than the sufentanil patients. During this period, the incidence of ischemia detected by ECG or echocardiography was not statistically different between desflurane (18 of 99) and sufentanil (9 of 98) groups. However, the duration and severity of ECG-detected myocardial ischemia was significantly less in the desflurane group. The incidence of myocardial ischemia after cardiopulmonary bypass and in the ICU did not differ between groups.
Geriatric Surgery
- Desflurane (desflurane, USP) plus N2O was compared to isoflurane plus N2O in a multicenter study (6 sites) of 203 ASA physical status II or III elderly patients, aged 57-91 years (median 71).
Induction
- Most patients were premedicated with fentanyl (mean 2 µg/kg), preoxygenated, and received thiopental (mean 4.3 mg/kg IV) or thiamylal (mean 4 mg/kg IV) followed by succinylcholine (mean 1.4 mg/kg IV) for intubation.
Maintenance & Recovery
- Heart rate and arterial blood pressure remained within 20% of preinduction baseline values during administration of Desflurane (desflurane, USP) 0.5-7.7% (average 3.6%) with 50-60% N2O. Induction, maintenance, and recovery cardiovascular measurements did not differ from those during isoflurane/N2O administration nor did the postoperative incidence of nausea and vomiting differ. The most common cardiovascular adverse event was hypotension occurring in 8% of the desflurane patients and 6% of the isoflurane patients.
Neurosurgery
- Desflurane (desflurane, USP) was studied in 38 patients aged 26-76 years (median 48 years), ASA physical status II or III undergoing neurosurgical procedures for intracranial lesions.
Induction
- Induction consisted of standard neuroanesthetic techniques including hyperventilation and thiopental.
Maintenance
- No change in cerebrospinal fluid pressure (CSFP) was observed in 8 patients who had intracranial tumors when the dose of Desflurane (desflurane, USP) was 0.5 MAC in N2O 50%. In another study of 9 patients with intracranial tumors, 0.8 MAC desflurane/air/O2 did not increase CSFP above post induction baseline values. In a different study of 10 patients receiving 1.1 MAC desflurane/air/O2, CSFP increased 7 mm Hg (range 3-13 mm Hg increase, with final values of 11-26 mm Hg) above the pre-drug values.
- All volatile anesthetics may increase intracranial pressure in patients with intracranial space occupying lesions. In such patients, Desflurane (desflurane, USP) should be administered at 0.8 MAC or less, and in conjunction with a barbiturate induction and hyperventilation (hypocapnia) in the period before cranial decompression. Appropriate attention must be paid to maintain cerebral perfusion pressure. The use of a lower dose of Desflurane (desflurane, USP) and the administration of a barbiturate and mannitol would be predicted to lessen the effect of desflurane on CSFP.
- Under hypocapnic conditions (PaCO2 27 mm Hg) Desflurane (desflurane, USP) 1 and 1.5 MAC did not increase cerebral blood flow (CBF) in 9 patients undergoing craniotomies. CBF reactivity to increasing PaCO2 from 27 to 35 mm Hg was also maintained at 1.25 MAC desflurane/air/O2.
Pediatric Surgery
- In a clinical safety trial conducted in children aged 2 to 16 years (mean 7.4 years), following induction with another agent, Desflurane (desflurane, USP) and isoflurane (in N2O/O2) were compared when delivered via face mask or laryngeal mask airway (LMA™ mask) for maintenance of anesthesia, after induction with intravenous propofol or inhaled sevoflurane, in order to assess the relative incidence of respiratory adverse events.
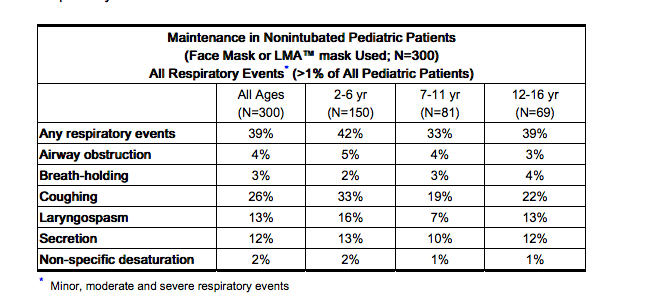
- Desflurane (desflurane, USP) was associated with higher rates (compared with isoflurane) of coughing, laryngospasm and secretions with an overall rate of respiratory events of 39%. Of the pediatric patients exposed to desflurane, 5% experienced severe laryngospasm (associated with significant desaturation; i.e. SpO2 of <90% for >15 seconds, or requiring succinylcholine), across all ages, 2-16 years old. Individual age group incidences of severe laryngospasm were 9% for 2-6 years old, 1% for 7-11 years old, and 1% for 12-16 years old. Removal of LMA™ mask under deep anesthesia (MAC range 0.6 – 2.3 with a mean of 1.12 MAC) was associated with a further increase in frequency of respiratory adverse events as compared to awake LMA™ mask removal or LMA™ mask removal under deep anesthesia with the comparator. The frequency and severity of non-respiratory adverse events were comparable between the two groups.
- The incidence of respiratory events under these conditions was highest in children aged 2-6 years. Therefore, similar studies in children under the age of 2 years were not initiated.
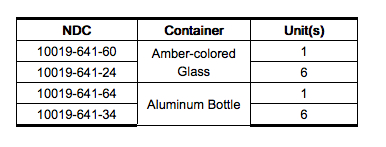
How Supplied
- Desflurane (desflurane, USP) is available in an amber-colored glass bottle or an aluminum bottle containing 240 mL of desflurane as follows:
Storage
- Store at room temperature, 15°-30°C (59°-86°F). Desflurane (desflurane, USP) has been demonstrated to be stable for the period defined by the expiration dating on the label. The bottle should be recapped after each use of Desflurane (desflurane, USP).
Images
Drug Images
{{#ask: Page Name::Desflurane |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Desflurane |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Anesthesia providers need to obtain the following information from patients prior to administration of anesthesia
- Medications they are taking, including herbal supplements
- Drug allergies, including allergic reactions to anesthetic agents (including hepatic sensitivity)
- Any history of severe reactions to prior administration of anesthetic
- If the patient or a member of the patient’s family has a history of malignant hyperthermia or if the patient has a history of Duchenne muscular dystrophy or other latent neuromuscular disease
Anesthesia providers should inform patients of the risks associated with Desflurane (desflurane, USP)
- Post-operative nausea and vomiting and respiratory adverse effects including coughing.
- There is no information of the effects of Desflurane (desflurane, USP) following anesthesia on the ability to operate an automobile or other heavy machinery. However, patients should be advised that the ability to perform such tasks may be impaired after receiving anesthetic agents.
- Baxter and Suprane are registered trademarks of Baxter International Inc.
- LMA is a trademark of The Laryngeal Mask Company Limited.
- Baxter logo
- Manufactured for
- Baxter Healthcare Corporation
- Deerfield, IL 60015 USA
- Revised 11/2013
- 07-19-72-483
Precautions with Alcohol
Alcohol-Desflurane interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Desflurane Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Desflurane Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Desflurane |Label Name=DESFLURANE Label.png
}}