Siponimod
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Zach Leibowitz [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Siponimod is a sphingosine 1-phosphate receptor modulator that is FDA approved for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults. Common adverse reactions include headache, hypertension, and transaminase increases.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Siponimod is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
Dosage
- The recommended maintenance dosage is 2 mg
- The recommended maintenance dosage in patients with a CYP2C9*1/*3 or *2/*3 genotype is 1 mg
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding siponimod Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding siponimod Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding siponimod Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding siponimod Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
Siponimod is contraindicated in patients who have:
- A CYP2C9*3/*3 genotype
- In the last 6 months experienced myocardial infarction, unstable angina, stroke, TIA, decompensated heart failure requiring hospitalization, or Class III or IV heart failure
- Presence of Mobitz type II second-degree, third-degree AV block, or sick sinus syndrome, unless patient has a functioning pacemaker
Warnings
Infections
Risk of Infections
- Siponimod causes a dose-dependent reduction in peripheral lymphocyte count to 20%-30% of baseline values because of reversible sequestration of lymphocytes in lymphoid tissues. Siponimod may therefore increase the risk of infections, some serious in nature. Life-threatening and rare fatal infections have occurred in association with siponimod.
- In Study 1 [see Clinical Studies ], the overall rate of infections was comparable between the siponimod-treated patients and those on placebo (49.0% vs. 49.1% respectively). However, herpes zoster, herpes infection, bronchitis, sinusitis, upper respiratory infection, and fungal skin infection were more common in siponimod-treated patients. In Study 1, serious infections occurred at a rate of 2.9% in siponimod-treated patients compared to 2.5% of patients receiving placebo.
- Before initiating treatment with siponimod, results from a recent complete blood count (i.e., within 6 months or after discontinuation of prior therapy) should be reviewed.
- Initiation of treatment with siponimod should be delayed in patients with severe active infection until resolution. Because residual pharmacodynamic effects, such as lowering effects on peripheral lymphocyte count, may persist for up to 3-4 weeks after discontinuation of siponimod, vigilance for infection should be continued throughout this period.
- Effective diagnostic and therapeutic strategies should be employed in patients with symptoms of infection while on therapy. Suspension of treatment with siponimod should be considered if a patient develops a serious infection.
Cryptococcal Infections
- Cases of fatal cryptococcal meningitis (CM) and disseminated cryptococcal infections have been reported with another sphingosine 1-phosphate (S1P) receptor modulator. Rare cases of CM have also occurred with siponimod. Physicians should be vigilant for clinical symptoms or signs of CM. Patients with symptoms or signs consistent with a cryptococcal infection should undergo prompt diagnostic evaluation and treatment. Siponimod treatment should be suspended until a cryptococcal infection has been excluded. If CM is diagnosed, appropriate treatment should be initiated.
Herpes Viral Infections
- Cases of herpes viral infection, including one case of reactivation of VZV infection leading to varicella zoster meningitis, have been reported in the development program of siponimod. In Study 1, the rate of herpetic infections was 4.6% in siponimod-treated patients compared to 3.0% of patients receiving placebo. In Study 1, an increase in the rate of herpes zoster infections was reported in 2.5% of siponimod-treated patients compared to 0.7% of patients receiving placebo. Patients without a healthcare professional confirmed history of varicella (chickenpox) or without documentation of a full course of vaccination against VZV should be tested for antibodies to VZV before initiating siponimod (see Vaccinations below).
Progressive Multifocal Leukoencephalopathy
- Progressive multifocal leukoencephalopathy (PML) is an opportunistic viral infection of the brain caused by the JC virus (JCV) that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.
- No cases of PML have been reported in siponimod-treated patients in the development program; however, PML has been reported in patients treated with a S1P receptor modulator and other multiple sclerosis (MS) therapies and has been associated with some risk factors (e.g., immunocompromised patients, polytherapy with immunosuppressants). Physicians should be vigilant for clinical symptoms or MRI findings that may be suggestive of PML. MRI findings may be apparent before clinical signs or symptoms. If PML is suspected, treatment with siponimod should be suspended until PML has been excluded.
Prior and Concomitant Treatment with Anti-neoplastic, Immune-Modulating, or Immunosuppressive Therapies
- Anti-neoplastic, immune-modulating, or immunosuppressive therapies (including corticosteroids) should be coadministered with caution because of the risk of additive immune system effects during such therapy.
Vaccinations
- Patients without a healthcare professional confirmed history of chickenpox or without documentation of a full course of vaccination against VZV should be tested for antibodies to VZV before initiating siponimod treatment. A full course of vaccination for antibody-negative patients with varicella vaccine is recommended prior to commencing treatment with siponimod, following which initiation of treatment with siponimod should be postponed for 4 weeks to allow the full effect of vaccination to occur.
- The use of live attenuated vaccines should be avoided while patients are taking siponimod and for 4 weeks after stopping treatment.
- Vaccinations may be less effective if administered during siponimod treatment. Siponimod treatment discontinuation 1 week prior to and until 4 weeks after a planned vaccination is recommended.
Macular Edema
- Macular edema was reported in 1.8% of siponimod-treated patients compared to 0.2% of patients receiving placebo. The majority of cases occurred within the first four months of therapy.
- An ophthalmic evaluation of the fundus, including the macula, is recommended in all patients before starting treatment and at any time if there is any change in vision while taking siponimod.
- Continuation of siponimod therapy in patients with macular edema has not been evaluated. A decision on whether or not siponimod should be discontinued needs to take into account the potential benefits and risks for the individual patient.
Macular Edema in Patients with a History of Uveitis or Diabetes Mellitus
- Patients with a history of uveitis and patients with diabetes mellitus are at increased risk of macular edema during siponimod therapy. The incidence of macular edema is also increased in MS patients with a history of uveitis. In the clinical trial experience in adult patients with all doses of siponimod, the rate of macular edema was approximately 10% in MS patients with a history of uveitis or diabetes mellitus versus 2% in those without a history of these diseases. In addition to the examination of the fundus, including the macula, prior to treatment, MS patients with diabetes mellitus or a history of uveitis should have regular follow-up examinations.
Bradyarrhythmia and Atrioventricular Conduction Delays
- Since initiation of siponimod treatment results in a transient decrease in heart rate and atrioventricular conduction delays, an up-titration scheme should be used to reach the maintenance dosage of siponimod.
- Siponimod was not studied in patients who had:
- In the last 6 months experienced myocardial infarction, unstable angina, stroke, TIA, or decompensated heart failure requiring hospitalization
- New York Heart Association Class II-IV heart failure
- Cardiac conduction or rhythm disorders, including complete left bundle branch block, sinus arrest or sino-atrial block, symptomatic bradycardia, sick sinus syndrome, Mobitz type II second degree AV-block or higher grade AV-block (either history or observed at screening), unless patient has a functioning pacemaker
- Significant QT prolongation (QTc greater than 500 msec)
- Arrhythmias requiring treatment with Class Ia or Class III anti-arrhythmic drugs
Reduction in Heart Rate
- After the first titration dose of siponimod, the heart rate decrease starts within an hour, and the Day 1 decline is maximal at approximately 3-4 hours. With continued up-titration, further heart rate decreases are seen on subsequent days, with maximal decrease from Day 1-baseline reached on Day 5-6. The highest daily post-dose decrease in absolute hourly mean heart rate is observed on Day 1, with the pulse declining on average 5-6 bpm. Post-dose declines on the following days are less pronounced. With continued dosing, heart rate starts increasing after Day 6 and reaches placebo levels within 10 days after treatment initiation.
- In Study 1, bradycardia occurred in 4.4% of siponimod-treated patients compared to 2.9% of patients receiving placebo. Patients who experienced bradycardia were generally asymptomatic. Few patients experienced symptoms, including dizziness or fatigue, and these symptoms resolved within 24 hours without intervention. Heart rates below 40 bpm were rarely observed.
Atrioventricular Conduction Delays
- Initiation of siponimod treatment has been associated with transient atrioventricular conduction delays that follow a similar temporal pattern as the observed decrease in heart rate during dose titration. The AV conduction delays manifested in most of the cases as first-degree AV block (prolonged PR interval on ECG), which occurred in 5.1% of siponimod-treated patients and in 1.9% of patients receiving placebo in Study 1. Second-degree AV blocks, usually Mobitz type I (Wenckebach), have been observed at the time of treatment initiation with siponimod in less than 1.7% of patients in clinical trials. The conduction abnormalities typically were transient, asymptomatic, resolved within 24 hours, rarely required treatment with atropine, and did not require discontinuation of siponimod treatment.
- If treatment with siponimod is considered, advice from a cardiologist should be sought:
- In patients with significant QT prolongation (QTc greater than 500 msec)
- In patients with arrhythmias requiring treatment with Class Ia or Class III anti-arrhythmic drugs
- In patients with ischemic heart disease, heart failure, history of cardiac arrest or myocardial infarction, cerebrovascular disease, and uncontrolled hypertension
- In patients with a history of second-degree Mobitz type II or higher AV block, sick-sinus syndrome, or sino-atrial heart block
Treatment-Initiation Recommendations
- Obtain an ECG in all patients to determine whether preexisting conduction abnormalities are present.
- In all patients, a dose titration is recommended for initiation of siponimod treatment to help reduce cardiac effects.
- In patients with sinus bradycardia (HR less than 55 bpm), first- or second-degree [Mobitz type I] AV block, or a history of myocardial infarction or heart failure with onset > 6 months prior to initiation, ECG testing and first-dose monitoring is recommended.
- Since significant bradycardia may be poorly tolerated in patients with history of cardiac arrest, cerebrovascular disease, uncontrolled hypertension, or severe untreated sleep apnea, siponimod is not recommended in these patients. If treatment is considered, advice from a cardiologist should be sought prior to initiation of treatment in order to determine the most appropriate monitoring strategy.
- Use of siponimod in patients with a history of recurrent syncope or symptomatic bradycardia should be based on an overall benefit-risk assessment. If treatment is considered, advice from a cardiologist should be sought prior to initiation of treatment in order to determine the most appropriate monitoring.
- Experience with siponimod is limited in patients receiving concurrent therapy with drugs that decrease heart-rate (e.g., beta-blockers, calcium channel blockers - diltiazem and verapamil, and other drugs that may decrease heart rate, such as ivabradine and digoxin). Concomitant use of these drugs during siponimod initiation may be associated with severe bradycardia and heart block.
- For patients receiving a stable dose of a beta-blocker, the resting heart rate should be considered before introducing siponimod treatment. If the resting heart rate is greater than 50 bpm under chronic beta-blocker treatment, siponimod can be introduced. If resting heart rate is less than or equal to 50 bpm, beta-blocker treatment should be interrupted until the baseline heart-rate is greater than 50 bpm. Treatment with siponimod can then be initiated and treatment with a beta-blocker can be reinitiated after siponimod has been up-titrated to the target maintenance dosage.
- For patients taking other drugs that decrease heart rate, treatment with siponimod should generally not be initiated without consultation from a cardiologist because of the potential additive effect on heart rate.
Missed Dose During Treatment Initiation and Reinitiation of Therapy Following Interruption
- If a titration dose is missed or if 4 or more consecutive daily doses are missed during maintenance treatment, reinitiate Day 1 of the dose titration and follow titration monitoring recommendations.
Respiratory Effects
- Dose-dependent reductions in absolute forced expiratory volume over 1 second (FEV1) were observed in siponimod-treated patients as early as 3 months after treatment initiation. In a placebo-controlled trial in adult patients, the decline in absolute FEV1 from baseline compared to placebo was 88 mL [95% confidence interval (CI): 139, 37] at 2 years. The mean difference between siponimod-treated patients and patients receiving placebo in percent predicted FEV1 at 2 years was 2.8% (95% CI: -4.5, -1.0). There is insufficient information to determine the reversibility of the decrease in FEV1 after drug discontinuation. In Study 1, five patients discontinued siponimod because of decreases in pulmonary function testing. Siponimod has been tested in MS patients with mild to moderate asthma and chronic obstructive pulmonary disease. The changes in FEV1 were similar in this subgroup compared with the overall population. Spirometric evaluation of respiratory function should be performed during therapy with siponimod if clinically indicated.
Liver Injury
- Elevations of transaminases may occur in siponimod-treated patients. Recent (i.e., within last 6 months) transaminase and bilirubin levels should be reviewed before initiation of siponimod therapy.
- In Study 1, elevations in transaminases and bilirubin were observed in 10.1% of siponimod-treated patients compared to 3.7% of patients receiving placebo, mainly because of transaminase [alanine aminotransferase/aspartate aminotransferase/gamma-glutamyltransferase (ALT/AST/GGT)] elevations.
- In Study 1, ALT or AST increased to three and five times the upper limit of normal (ULN) in 5.6% and 1.4% of siponimod-treated patients, respectively, compared to 1.5% and 0.5% of patients receiving placebo, respectively. ALT or AST increased eight and ten times ULN in siponimod-treated patients (0.5% and 0.2%, respectively) compared to no patients receiving placebo. The majority of elevations occurred within 6 months of starting treatment. ALT levels returned to normal within approximately 1 month after discontinuation of siponimod. In clinical trials, siponimod was discontinued if the elevation exceeded a 3-fold increase and the patient showed symptoms related to hepatic dysfunction.
- Patients who develop symptoms suggestive of hepatic dysfunction, such as unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, rash with eosinophilia, or jaundice and/or dark urine during treatment, should have liver enzymes checked. siponimod should be discontinued if significant liver injury is confirmed.
- Although there are no data to establish that patients with preexisting liver disease are at increased risk to develop elevated liver function test values when taking siponimod, caution should be exercised when using siponimod in patients with a history of significant liver disease.
Increased Blood Pressure
- In Study 1, siponimod-treated patients had an average increase over placebo of approximately 3 mmHg in systolic pressure and 1.2 mmHg in diastolic pressure, which was first detected after approximately 1 month of treatment initiation and persisted with continued treatment. Hypertension was reported as an adverse reaction in 12.5% of siponimod-treated patients and in 9.2% of patients receiving placebo. Blood pressure should be monitored during treatment with siponimod and managed appropriately.
Fetal Risk
- Based on animal studies, siponimod may cause fetal harm. Because it takes approximately 10 days to eliminate siponimod from the body, women of childbearing potential should use effective contraception to avoid pregnancy during and for 10 days after stopping siponimod treatment.
Posterior Reversible Encephalopathy Syndrome
- Rare cases of posterior reversible encephalopathy syndrome (PRES) have been reported in patients receiving a sphingosine 1-phosphate (S1P) receptor modulator. Such events have not been reported for siponimod-treated patients in the development program. However, should a siponimod-treated patient develop any unexpected neurological or psychiatric symptoms/signs (e.g., cognitive deficits, behavioral changes, cortical visual disturbances, or any other neurological cortical symptoms/signs), any symptom/sign suggestive of an increase of intracranial pressure, or accelerated neurological deterioration, the physician should promptly schedule a complete physical and neurological examination and should consider a MRI. Symptoms of PRES are usually reversible but may evolve into ischemic stroke or cerebral hemorrhage. Delay in diagnosis and treatment may lead to permanent neurological sequelae. If PRES is suspected, siponimod should be discontinued.
Unintended Additive Immunosuppressive Effects From Prior Treatment With Immunosuppressive or Immune-Modulating Therapies
- When switching from drugs with prolonged immune effects, the half-life and mode of action of these drugs must be considered to avoid unintended additive immunosuppressive effects while at the same time minimizing risk of disease reactivation, when initiating siponimod.
- Initiating treatment with siponimod after treatment with alemtuzumab is not recommended.
Severe Increase in Disability After Stopping siponimod
- Severe exacerbation of disease, including disease rebound, has been rarely reported after discontinuation of a S1P receptor modulator. The possibility of severe exacerbation of disease should be considered after stopping siponimod treatment. Patients should be observed for a severe increase in disability upon siponimod discontinuation and appropriate treatment should be instituted, as required.
Immune System Effects After Stopping siponimod
- After stopping siponimod therapy, siponimod remains in the blood for up to 10 days. Starting other therapies during this interval will result in concomitant exposure to siponimod.
- Lymphocyte counts returned to the normal range in 90% of patients within 10 days of stopping therapy. However, residual pharmacodynamics effects, such as lowering effects on peripheral lymphocyte count, may persist for up to 3-4 weeks after the last dose. Use of immunosuppressants within this period may lead to an additive effect on the immune system, and therefore caution should be applied 3-4 weeks after the last dose of siponimod.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- A total of 1737 MS patients have received siponimod at doses of at least 2 mg daily. These patients were included in Study 1 and in a Phase 2 placebo-controlled study in patients with MS. In Study 1, 67% of siponimod-treated patients completed the double-blind part of the study, compared to 59.0% of patients receiving placebo. Adverse events led to discontinuation of treatment in 8.5% of siponimod-treated patients, compared to 5.1% of patients receiving placebo. The most common adverse reactions (incidence at least 10%) in siponimod-treated patients in Study 1 were headache, hypertension, and transaminase increases.
- Table 3 lists adverse reactions that occurred in at least 5% of siponimod-treated patients and at a rate at least 1% higher than in patients receiving placebo.

- The following adverse reactions have occurred in less than 5% of siponimod-treated patients but at a rate at least 1% higher than in patients receiving placebo: herpes zoster, lymphopenia, seizure, tremor, macular edema, AV block (1st and 2nd degree), asthenia, and pulmonary function test decreased.
Seizures
- In Study 1, cases of seizures were reported in 1.7% of siponimod-treated patients, compared to 0.4% in patients receiving placebo. It is not known whether these events were related to the effects of MS, to siponimod, or to a combination of both.
Respiratory Effects
- Dose-dependent reductions in forced expiratory volume over 1 second (FEV1) were observed in patients treated with siponimod
Vascular Events
- Vascular events, including ischemic strokes, pulmonary embolisms, and myocardial infarctions, were reported in 3.0% of siponimod-treated patients compared to 2.6% of patients receiving placebo. Some of these events were fatal. Physicians and patients should remain alert for the development of vascular events throughout treatment, even in the absence of previous vascular symptoms. Patients should be informed about the symptoms of cardiac or cerebral ischemia caused by vascular events and the steps to take if they occur.
Malignancies
- Malignancies such as malignant melanoma in situ and seminoma were reported in siponimod-treated patients in Study 1. An increased risk of cutaneous malignancies has been reported in association with another S1P modulator.
Postmarketing Experience
There is limited information regarding Siponimod Postmarketing Experience in the drug label.
Drug Interactions
Anti-Neoplastic, Immune-Modulating, or Immunosuppressive Therapies
- Siponimod has not been studied in combination with anti-neoplastic, immune-modulating, or immunosuppressive therapies. Caution should be used during concomitant administration because of the risk of additive immune effects during such therapy and in the weeks following administration.
- When switching from drugs with prolonged immune effects, the half-life and mode of action of these drugs must be considered in order to avoid unintended additive immunosuppressive effects.
- Because of the characteristics and duration of alemtuzumab immune suppressive effects, initiating treatment with siponimod after alemtuzumab is not recommended.
- Siponimod can generally be started immediately after discontinuation of beta interferon or glatiramer acetate.
Anti-Arrhythmic Drugs, QT Prolonging Drugs, Drugs That May Decrease Heart Rate
- Siponimod has not been studied in patients taking QT prolonging drugs.
- Class Ia (e.g., quinidine, procainamide) and Class III (e.g., amiodarone, sotalol) anti-arrhythmic drugs have been associated with cases of Torsades de Pointes in patients with bradycardia. If treatment with siponimod is considered, advice from a cardiologist should be sought.
- Because of the potential additive effects on heart rate, treatment with siponimod should generally not be initiated in patients who are concurrently treated with QT prolonging drugs with known arrhythmogenic properties, heart rate lowering calcium channel blockers (e.g., verapamil, diltiazem), or other drugs that may decrease heart rate (e.g., ivabradine, digoxin). If treatment with siponimod is considered, advice from a cardiologist should be sought regarding the switch to non-heart-rate lowering drugs or appropriate monitoring for treatment initiation.
Beta-Blockers
- Caution should be applied when siponimod is initiated in patients receiving treatment with a beta-blocker because of the additive effects on lowering heart rate; temporary interruption of the beta-blocker treatment may be needed prior to initiation of siponimod. Beta-blocker treatment can be initiated in patients receiving stable doses of siponimod.
Vaccination
- During and for up to one month after discontinuation of treatment with siponimod, vaccinations may be less effective; therefore siponimod treatment should be paused 1 week prior and for 4 weeks after vaccination.
- The use of live attenuated vaccines may carry the risk of infection and should therefore be avoided during siponimod treatment and for up to 4 weeks after discontinuation of treatment with siponimod.
CYP2C9 and CYP3A4 Inhibitors
- Because of a significant increase in exposure to siponimod, concomitant use of siponimod and drugs that cause moderate CYP2C9 and moderate or strong CYP3A4 inhibition is not recommended. This concomitant drug regimen can consist of a moderate CYP2C9/CYP3A4 dual inhibitor (e.g., fluconazole) or a moderate CYP2C9 inhibitor in combination with a separate - moderate or strong CYP3A4 inhibitor.
- Caution should be exercised for concomitant use of siponimod with moderate CYP2C9 inhibitors.
CYP2C9 and CYP3A4 Inducers
- Because of a significant decrease in siponimod exposure, concomitant use of siponimod and drugs that cause moderate CYP2C9 and strong CYP3A4 induction is not recommended for all patients. This concomitant drug regimen can consist of moderate CYP2C9/strong CYP3A4 dual inducer (e.g., rifampin or carbamazepine) or a moderate CYP2C9 inducer in combination with a separate strong CYP3A4 inducer.
- Caution should be exercised for concomitant use of siponimod with moderate CYP2C9 inducers.
- Concomitant use of siponimod and moderate (e.g., modafinil, efavirenz) or strong CYP3A4 inducers is not recommended for patients with CYP2C9*1/*3 and*2/*3 genotype.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- There are no adequate data on the developmental risk associated with the use of siponimod in pregnant women. Based on animal data and its mechanism of action, siponimod can cause fetal harm when administered to a pregnant woman (see Data). Reproductive and developmental studies in pregnant rats and rabbits have demonstrated siponimod-induced embryotoxicity and fetotoxicity in rats and rabbits and teratogenicity in rats. Increased incidences of post-implantation loss and fetal abnormalities (external, urogenital and skeletal) in rat and of embryo-fetal deaths, abortions and fetal variations (skeletal and visceral) in rabbit were observed following prenatal exposure to siponimod starting at a dose 2 times the exposure in humans at the highest recommended dose of 2 mg/day.
- In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Animal Data
- When siponimod (0, 1, 5, or 40 mg/kg) was orally administered to pregnant rats during the period of organogenesis, post implantation loss and fetal malformations (visceral and skeletal) were increased at the lowest dose tested, the only dose with fetuses available for evaluation. A no-effect dose for adverse effects on embryo-fetal development in rats was not identified. Plasma exposure AUC at the lowest dose tested was approximately 18 times that in humans at the recommended human dose (RHD) of 2 mg/day.
- When siponimod (0, 0.1, 1, or 5 mg/kg) was orally administered to pregnant rabbits during the period of organogenesis, embryolethality and increased incidences of fetal skeletal variations were observed at all but the lowest dose tested. Plasma exposure (AUC) at the no-effect dose (0.1 mg/kg) for adverse effects on embryo-fetal development in rabbits is less that than in humans at the RHD.
- When siponimod (0, 0.05, 0.15, or 0.5 mg/kg) was orally administered to female rats throughout pregnancy and lactation, increased mortality, decreased body weight, and delayed sexual maturation were observed in the offspring at all but the lowest dose tested. An increase in malformations was observed at all doses. A no-effect dose for adverse effects on pre- and postnatal development in rats was not identified. The lowest dose tested (0.05 mg/kg) is less than the RHD, on a mg/m2 basis.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Siponimod in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Siponimod during labor and delivery.
Nursing Mothers
- There are no data on the presence of siponimod in human milk, the effects of siponimod on the breastfed infant, or the effects of the drug on milk production. A study in lactating rats has shown excretion of siponimod and/or its metabolites in milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for siponimod and any potential adverse effects on the breastfed infant from siponimod or from the underlying maternal condition.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- Clinical studies of siponimod did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Siponimod with respect to specific gender populations.
Race
There is no FDA guidance on the use of Siponimod with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Siponimod in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Siponimod in patients with hepatic impairment.
Females of Reproductive Potential and Males
- Before initiation of siponimod treatment, women of childbearing potential should be counselled on the potential for a serious risk to the fetus and the need for effective contraception during treatment with siponimod. Since it takes approximately 10 days to eliminate the compound from the body after stopping treatment, the potential risk to the fetus may persist and women should use effective contraception during this period.
Immunocompromised Patients
There is no FDA guidance one the use of Siponimod in patients who are immunocompromised.
CYP2C9 Genotype
Before initiation of treatment with siponimod, test patients to determine CYP2C9 genotype. Siponimod is contraindicated in patients homozygous for CYP2C9*3 (i.e., CYP2C9*3/*3 genotype), which is approximately 0.4%-0.5% of Caucasians and less in others, because of substantially elevated siponimod plasma levels. Siponimod dosage adjustment is recommended in patients with CYP2C9*1/*3 or *2/*3 genotype because of an increase in exposure to siponimod.
Administration and Monitoring
Administration
Assessments Prior to First Dose of Siponimod
Before initiation of treatment with siponimod, assess the following: CYP2C9 Genotype Determination
- Test patients for CYP2C9 variants to determine CYP2C9 genotype. An FDA-cleared or -approved test for the detection of CYP2C9 variants to direct the use of siponimod is not currently available.
Complete Blood Count
- Review results of a recent complete blood count (CBC).
Ophthalmic Evaluation
- Obtain an evaluation of the fundus, including the macula
Cardiac Evaluation
- Obtain an electrocardiogram (ECG) to determine whether preexisting conduction abnormalities are present. In patients with certain preexisting conditions, advice from a cardiologist and first-dose monitoring is recommended.
- Determine whether patients are taking drugs that could slow heart rate or atrioventricular (AV) conduction.
Current or Prior Medications
- If patients are taking anti-neoplastic, immunosuppressive, or immune-modulating therapies, or if there is a history of prior use of these drugs, consider possible unintended additive immunosuppressive effects before initiating treatment with siponimod.
Vaccinations
- Test patients for antibodies to varicella zoster virus (VZV) before initiating siponimod; VZV vaccination of antibody-negative patients is recommended prior to commencing treatment with siponimod.
Liver Function Tests
- Obtain recent (i.e., within last 6 months) transaminase and bilirubin levels
Recommended Dosage in Patients With CYP2C9 Genotypes *1/*1, *1/*2, or *2/*2
Maintenance Dosage
- After treatment titration (see Treatment Initiation), the recommended maintenance dosage of siponimod is 2 mg taken orally once daily starting on Day 6. Dosage adjustment is required in patients with a CYP2C9*1/*3 or *2/*3 genotype.
Treatment Initiation
- Initiate siponimod with a 5-day titration, as shown in Table 1. A starter pack should be used for patients who will be titrated to the 2-mg maintenance dosage

- If one titration dose is missed for more than 24 hours, treatment needs to be reinitiated with Day 1 of the titration regimen.
Recommended Dosage in Patients With CYP2C9 Genotypes *1/*3 or *2/*3
Maintenance Dosage
- In patients with a CYP2C9*1/*3 or *2/*3 genotype, after treatment titration (see Treatment Initiation), the recommended maintenance dosage of siponimod is 1 mg taken orally once daily starting on Day 5.
Treatment Initiation
- Initiate siponimod with a 4-day titration, as shown in Table 2. Do not use the starter pack for patients who will be titrated to the 1-mg maintenance dosage.

- If one titration dose is missed for more than 24 hours, treatment needs to be reinitiated with Day 1 of the titration regimen.
Monitoring
First Dose Monitoring in Patients With Certain Preexisting Cardiac Conditions
- Because initiation of siponimod treatment results in a decrease in heart rate (HR), first-dose 6 hour monitoring is recommended for patients with sinus bradycardia [HR less than 55 beats per minute (bpm)], first- or second-degree [Mobitz type I] AV block, or a history of myocardial infarction or heart failure.
First Dose 6-Hour Monitoring
- Administer the first dose of siponimod in a setting where resources to appropriately manage symptomatic bradycardia are available. Monitor patients for 6 hours after the first dose for signs and symptoms of bradycardia with hourly pulse and blood pressure measurement. Obtain an ECG in these patients at the end of the Day 1 observation period.
Additional Monitoring After 6-Hour Monitoring
- If any of the following abnormalities are present after 6 hours (even in the absence of symptoms), continue monitoring until the abnormality resolves:
- The heart rate 6 hours postdose is less than 45 ppm
- The heart rate 6 hours postdose is at the lowest value postdose, suggesting that the maximum pharmacodynamic effect on the heart may not have occurred
- The ECG 6 hours postdose shows new onset second-degree or higher AV block
- If postdose symptomatic bradycardia, bradyarrhythmia, or conduction related symptoms occur, or if ECG 6 hours post-dose shows new onset second degree or higher AV block or QTc greater than or equal to 500 msec, initiate appropriate management, begin continuous ECG monitoring, and continue monitoring until the symptoms have resolved if no pharmacological treatment is required. If pharmacological treatment is required, continue monitoring overnight and repeat 6-hour monitoring after the second dose.
- Advice from a cardiologist should be sought to determine the most appropriate monitoring strategy (which may include overnight monitoring) during treatment initiation, if treatment with siponimod is considered in patients:
- With some preexisting heart and cerebrovascular conditions
- With a prolonged QTc interval before dosing or during the 6-hour observation, or at additional risk for QT prolongation, or on concurrent therapy with QT prolonging drugs with a known risk of torsades de points
- Receiving concurrent therapy with drugs that slow heart rate or AV conduction
Reinitiation of Siponimod After Treatment Interruption
- After the initial titration is complete, if siponimod treatment is interrupted for 4 or more consecutive daily doses, reinitiate treatment with Day 1 of the titration regimen; also complete first-dose monitoring in patients for whom it is recommended.
IV Compatibility
There is limited information regarding the compatibility of Siponimod and IV administrations.
Overdosage
- In patients with overdosage of siponimod, it is important to observe for signs and symptoms of bradycardia, which may include overnight monitoring. Regular measurements of pulse rate and blood pressure are required, and ECGs should be performed.
- There is no specific antidote to siponimod available. Neither dialysis nor plasma exchange would result in meaningful removal of siponimod from the body. The decrease in heart rate induced by siponimod can be reversed by atropine or isoprenaline.
Pharmacology
| |
Siponimod
| |
| Systematic (IUPAC) name | |
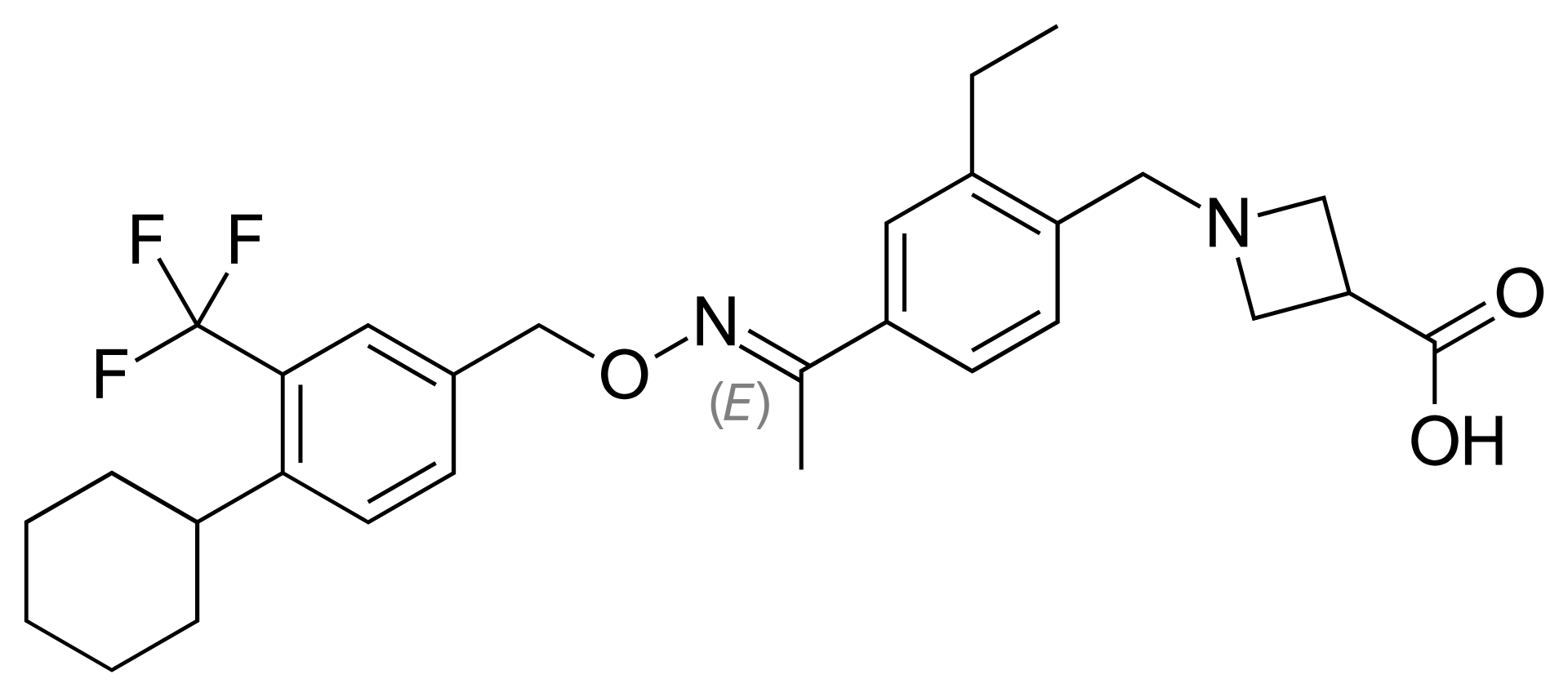
| 1-({4-[(1E)-1-({[4-Cyclohexyl- 3-(trifluoromethyl)phenyl]methoxy}imino)ethyl]-2-ethylphenyl}methyl)azetidine-3-carboxylic acid | |
| Identifiers | |
| CAS number | |
| ATC code | L04 |
| PubChem | |
| Chemical data | |
| Formula | C29H35F3N2O3 |
| Mol. mass | 516.26 g/mol |
| SMILES | & |
| Synonyms | BAF-312 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | By mouth |
Mechanism of Action
- Siponimod is a sphingosine-1-phosphate (S1P) receptor modulator. Siponimod binds with high affinity to S1P receptors 1 and 5. Siponimod blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. The mechanism by which siponimod exerts therapeutic effects in multiple sclerosis is unknown, but may involve reduction of lymphocyte migration into the central nervous system.
Structure

Pharmacodynamics
Immune System
- Siponimod induces a dose-dependent reduction of the peripheral blood lymphocyte count within 6 hours of the first dose, caused by the reversible sequestration of lymphocytes in lymphoid tissues.
- With continued daily dosing, the lymphocyte count continues to decrease, reaching a nadir median (90% CI) lymphocyte count of approximately 0.560 (0.271-1.08) cells/nL in a typical CYP2C9*1/*1 or *1/*2, non-Japanese patient, corresponding to 20% to 30% of baseline. Low lymphocyte counts are maintained with chronic daily dosing.
- Lymphocyte counts returned to the normal range in 90% of patients within 10 days of stopping therapy. After stopping siponimod treatment, residual lowering effects on peripheral lymphocyte count may persist for up to 3-4 weeks after the last dose.
Heart Rate and Rhythm
- Siponimod causes a transient reduction in heart rate and atrioventricular conduction upon treatment initiation. The maximum decline in heart rate is seen in the first 6 hours post dose. Autonomic responses of the heart, including diurnal variation of heart rate and response to exercise, are not affected by siponimod treatment.
- A transient, dose-dependent decrease in heart rate was observed during the initial dosing phase of siponimod, which plateaued at doses greater than or equal to 5 mg, and bradyarrhythmic events (AV blocks and sinus pauses) were detected at a higher incidence under siponimod treatment, compared to placebo.
- No second-degree AV blocks of Mobitz type II or higher degree were observed. Most AV blocks and sinus pauses occurred above the recommended dose of 2 mg, with notably higher incidence under non-titrated conditions compared to dose titration conditions.
- The decrease in heart rate induced by siponimod can be reversed by atropine or isoprenaline.
Beta-Blockers
- The negative chronotropic effect of coadministration of siponimod and propranolol was evaluated in a dedicated pharmacodynamics (PD)/safety study. The addition of propranolol on top of siponimod at steady-state had less pronounced negative chronotropic effects (less than additive effect) than the addition of siponimod to propranolol at steady state (additive HR effect)
Cardiac Electrophysiology
- In a thorough QT study with doses of 2 mg (recommended dose) and 10 mg (five times the recommended dose) siponimod at steady-state, siponimod treatment resulted in a prolongation of QTc , with the maximum mean (upper bound of the two-sided 90% CI) of 7.8 (9.93) ms at 2 mg dose and 7.2 (9.72) ms at 10 mg dose. There was an absence of dose- and exposure-response relationship for QTc effects with the 5-fold dose and exposures achieved by the supratherapeutic dose. No subject had absolute QTcF greater than 480 ms or ΔQTcF greater than 60 ms for siponimod treatment.
Pulmonary Function
- Dose-dependent reductions in absolute forced expiratory volume over 1 second were observed in siponimod-treated patients and were greater than in patients taking placebo.
Pharmacokinetics
- Siponimod concentration increases in an apparent dose-proportional manner after multiple once-daily doses of siponimod 0.3 mg to 20 mg. Steady-state plasma concentrations are reached after approximately 6 days of once-daily dosing, and steady-state levels are approximately 2-3-fold greater than the initial dose. An up-titration regimen is used to reach the clinical therapeutic dose of siponimod of 2 mg after 6 days, and 4 additional days of dosing are required to reach the steady-state-plasma concentrations.
Absorption
- The time (Tmax) to reach maximum plasma concentrations (Cmax) after oral administration of immediate release oral dosage forms of siponimod was about 4 hours (range 3-8 hours). Siponimod absorption is extensive (greater than or equal to 70%, based on the amount of radioactivity excreted in urine and the amount of metabolites in feces extrapolated to infinity). The absolute oral bioavailability of siponimod is approximately 84%. After administration of siponimod 2 mg once-daily over 10 days, a mean Cmax of 30.4 ng/mL and mean area under plasma concentration-time curve over dosing interval (AUCtau) of 558 h*ng/mL were observed on day 10. Steady-state was reached after approximately 6 days of once-daily administration of siponimod.
Food Effect
- Food intake resulted in delayed absorption (the median Tmax increased by approximately 2-3 hours). Food intake had no effect on the systemic exposure of siponimod (Cmax and AUC). Therefore, siponimod may be taken without regard to meals.
Distribution
- Siponimod distributes to body tissues with a moderate mean volume of distribution of 124 L. Siponimod fraction found in plasma is 68% in humans. Animal studies show that siponimod readily crosses the blood-brain-barrier. Protein binding of siponimod is greater than 99.9% in healthy subjects and in hepatic and renal impaired patients.
Elimination Metabolism
- Siponimod is extensively metabolized, mainly via CYP2C9 (79.3%), followed by CYP3A4 (18.5%). The pharmacological activity of the main metabolites M3 and M17 is not expected to contribute to the clinical effect and the safety of siponimod in humans.
Excretion
- An apparent systemic clearance (CL/F) of 3.11 L/h was estimated in MS patients. The apparent elimination half-life is approximately 30 hours.
- Siponimod is eliminated from the systemic circulation mainly due to metabolism, and subsequent biliary/fecal excretion. Unchanged siponimod was not detected in urine.
Specific Populations Male and Female Patients
- Gender has no influence on siponimod pharmacokinetics (PK).
Racial or Ethnic Groups
- The single-dose PK parameters were not different between Japanese and Caucasians healthy subjects, indicating absence of ethnic sensitivity on the PK of siponimod.
Patients with Renal Impairment
- No dose adjustments are needed in patients with renal impairment. Mean siponimod half-life and Cmax (total and unbound) were comparable between subjects with severe renal impairment and healthy subjects. Unbound AUCs were only slightly increased (by 33%), compared to healthy subjects, and it is not expected to be clinically significant. The effects of end-stage renal disease or hemodialysis on the PK of siponimod has not been studied. Due to the high plasma protein binding (greater than 99.9%) of siponimod, hemodialysis is not expected to alter the total and unbound siponimod concentration and no dose adjustments are anticipated based on these considerations.
Patients with Hepatic Impairment
- No dose adjustments for siponimod are needed in patients with hepatic impairment. The unbound siponimod AUC parameters are 15% and 50% higher in subjects with moderate and severe hepatic impairment, respectively, in comparison with healthy subjects for the 0.25 mg single dose studied. The increased unbound siponimod AUC in subjects with moderate and severe hepatic impairment is not expected to be clinically significant. The mean half-life of siponimod was unchanged in hepatic impairment.
Drug Interaction Studies Siponimod (and Metabolites M3, M17) as a Causative Agent of Interaction
- In vitro investigations indicated that siponimod and its major systemic metabolites M3 and M17 do not show any clinically relevant drug-drug interaction potential at the therapeutic dose of 2 mg once-daily for all investigated CYP enzymes and transporters.
Siponimod as an Object of Interaction
- CYP2C9 is polymorphic and the genotype influences the fractional contributions of the two oxidative metabolism pathways to overall elimination. Physiologically based PK modeling indicates a differential CYP2C9 genotype-dependent inhibition and induction of CYP3A4 pathways. With decreased CYP2C9 metabolic activity in the respective genotypes, a larger effect of the CYP3A4 perpetrators on siponimod exposure is anticipated.
Coadministration of Siponimod with CYP2C9 and CYP3A4 Inhibitors
- The coadministration of fluconazole (moderate CYP2C9 and CYP3A4 dual inhibitor) 200 mg daily at steady-state and a single dose of siponimod 4 mg in CYP2C9*1/*1 healthy volunteers led to a 2-fold increase in the AUC of siponimod. Mean siponimod terminal half-life was increased by 50%. Fluconazole led to a 2- to 4-fold increase in the AUCtau,ss of siponimod across different CYP2C9 genotypes, according to in silica evaluation.
Coadministration of siponimod with CYP2C9 and CYP3A4 Inducers
- The coadministration of siponimod 2 mg daily in the presence of 600 mg daily doses of rifampin (strong CYP3A4 and moderate CYP2C9 dual inducer) decreased siponimod AUCtau,ss and Cmax,ss by 57% and 45%, respectively in CY2C9*1/*1 subjects. Rifampin and efavirenz (moderate CYP3A4 inducer) reduced the AUCtau,ss of siponimod by up to 78% and up to 52%, respectively, across CYP2C9 genotypes, according to in silica evaluation.
Oral Contraceptives
- The effects of coadministration of siponimod 2 mg and 4 mg (twice the recommended dosage) once daily with a monophonic oral contraceptive (OC) containing 30 mcg ethinyl estradiol and 150 mcg levonorgestrel were assessed in 24 healthy female subjects (18 to 40 years of age; CYP2C9*1/*1 genotype). There were no clinically relevant effects on the PK or PD of the OC. No interaction studies have been performed with OCs containing other progestagens; however, an effect of siponimod on their exposure is not expected.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Oral carcinogenicity studies of spinomod were conducted in mice and rats. In mice administered siponimod (0, 2, 8, or 25 mg/kg/day) for up to 104 weeks, there was an increase in malignant lymphoma in females at all doses and in hemangiosarcoma and combined hemangioma and hemangiosarcoma at all doses in males and females. The lowest dose tested is approximately 5 times the recommended human dose (RHD) of 2 mg/day, on a body surface area (mg/m2) basis.
- In rats, administration of siponimod (0, 10, 30, or 90 mg/kg/day in males; 0, 3, 10, or 30 mg/kg/day in females) for up to 104 weeks, there was an increase in thyroid follicular cell adenoma and combined thyroid follicular cell adenoma and carcinoma in males at the highest dose tested. These findings are considered secondary to liver enzyme induction in rats and are not considered relevant to humans. Plasma siponimod exposure (AUC) at the highest dose tested is approximately 200 times that in humans at the RHD.
Mutagenesis
- Siponimod was negative in a battery of in vitro (Ames, chromosomal aberration in mammalian cells) and in vivo (micronucleus in mouse and rat) assays.
Impairment of Fertility
- When siponimod was administered orally (0, 2, 20, or 200 mg/kg) to male rats (mated with untreated females) prior to and throughout the mating period, there was a dose-related increase in precoital interval at all doses. A decrease in implantation sites, an increase in preimplantation loss, and a decrease in the number of viable fetuses were observed at the highest dose tested. The higher no-effect dose for adverse effects on fertility (20 mg/kg) is approximately 100 times the RHD on a mg/m2 basis.
- When siponimod was administered orally (0, 0.1, 0.3, or 1 mg/kg) to female rats (mated with untreated males) prior to and during mating, and continuing to Day 6 of gestation, no effects on fertility were observed up to the highest dose tested (1 mg/kg). Plasma siponimod exposure (AUC) at the highest dose tested is approximately 16 times that in humans at the RHD.
Clinical Studies
- The efficacy of siponimod was demonstrated in Study 1, a randomized, double-blind, parallel-group, placebo-controlled, time-to-event study in patients with secondary progressive multiple sclerosis (SPMS) who had evidence of disability progression in the prior 2 years, no evidence of relapse in 3 months prior to study enrollment, and an Expanded Disability Status Scale (EDSS) score of 3.0-6.5 at study entry (NCT 01665144).
- Patients were randomized to receive either once daily siponimod 2 mg or placebo, beginning with a dose titration. Evaluations were performed at screening, every 3 months during the study, and at the time of a suspected relapse. MRI evaluations were performed at screening and every 12 months.
- The primary endpoint of the study was the time to 3-month confirmed disability progression (CDP), defined as at least a 1-point increase from baseline in EDSS (0.5-point increase for patients with baseline EDSS of 5.5 or higher) sustained for 3 months. A prespecified hierarchical analysis consisted of the primary endpoint and 2 secondary endpoints, the time to 3-month confirmed worsening of at least 20% from baseline on the timed 25-foot walk test and the change from baseline in T2 lesion volume. Additional endpoints included annualized relapse rate (relapses/year) and MRI measures of inflammatory disease activity.
- Study duration was variable for individual patients (median study duration was 21 months, range 1 day-37 months).
- Study 1 randomized 1651 patients to either siponimod 2 mg (N = 1105) or placebo (N = 546); 82% of siponimod-treated patients and 78% of placebo-treated patients completed the study. Median age was 49.0 years, 95% of patients were white, and 60% female. The median disease duration was 16.0 years, and median EDSS score at baseline was 6.0 (56% of patients had ≥ 6.0 EDSS at baseline); 36% of patients had one or more relapses in the 2 years prior to study entry; 22% of those patients with available imaging had one or more gadolinium-enhancing lesions on their baseline MRI scan; 78% of patients had been previously treated with an MS therapy.
- Results are presented in Table 4. Siponimod was superior to placebo in reducing the risk of confirmed disability progression, based on a time-to-event analysis (hazard ratio 0.79, p < 0.0134; see Figure 1). Siponimod did not significantly delay the time to 20% deterioration in the timed 25-foot walk, compared to placebo. Patients treated with siponimod had a 55% relative reduction in annualized relapse rate, compared to patients on placebo (nominal p-value < 0.0001). The absolute reduction in the annualized relapse rate was 0.089. Although siponimod had a significant effect on disability progression compared to placebo in patients with active SPMS (e.g., SPMS patients with an MS relapse in the 2 years prior to the study), the effect of siponimod in patients with non-active SPMS was not statistically significant (see Figure 2).



How Supplied
Siponimod film-coated tablets are supplied as follows:
- Starter Pack* – blister card of twelve 0.25 mg tablets in a calendarized blister wallet
- Bottle of 28 tablets
- Bottle of 30 tablets
Storage
Unopened Containers
- Store unopened containers of siponimod 0.25 mg and 2 mg film-coated tablets in a refrigerator between 2°C to 8°C (36°F to 46°F).
Opened Containers: Starter Pack/Blister Card
- Siponimod 0.25 mg film-coated tablets in the Starter Pack may be stored at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature] for up to 1 week after opening the blister. Store in original container.
Bottles
- Siponimod 0.25 mg and 2 mg film-coated tablets in bottles may be stored at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature] for up to 1 month after opening the bottles.
Images
Drug Images


{{#ask: Page Name::Siponimod |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Siponimod |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
- Tell patients not to discontinue siponimod without first discussing this with the prescribing physician. Advise patients to contact their physician if they accidentally take more siponimod than prescribed.
Risk of Infections
- Inform patients that they may have an increased risk of infections, some of which could be life-threatening, when taking siponimod, and that they should contact their physician if they develop symptoms of infection. Advise patients that the use of some vaccines containing live virus (live attenuated vaccines) should be avoided during treatment with siponimod and siponimod should be paused 1 week prior and until 4 weeks after a planned vaccination. Recommend that patients postpone treatment with siponimod for at least 1 month after VZV vaccination. Inform patients that prior or concomitant use of drugs that suppress the immune system may increase the risk of infection.
Macular Edema
- Advise patients that siponimod may cause macular edema, and that they should contact their physician if they experience any changes in their vision while taking siponimod. Inform patients with diabetes mellitus or a history of uveitis that their risk of macular edema is increased.
Cardiac Effects
- Advise patients that initiation of siponimod treatment results in transient decrease in heart rate. Inform patients that to reduce this effect, dosage titration is required. Advise patients that dosage titration is also required if a dose is missed for more than 24 hours during the titration or if 4 or more consecutive daily maintenance doses are missed. Inform certain patients with certain pre-existing cardiac conditions that they will need to be observed in the doctor's office or other facility for at least 6 hours after the first dose and after reinitiation if treatment is interrupted or discontinued for certain periods.
Respiratory Effects
- Advise patients that they should contact their physician if they experience new onset or worsening of dyspnea.
Liver Injury
- Inform patients that siponimod may increase liver enzymes. Advise patient that they should contact their physician if they experience any unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine during treatment.
Pregnancy and Fetal Risk
- Inform patients that, based on animal studies siponimod may cause fetal harm. Discuss with women of childbearing age whether they are pregnant, might be pregnant, or are trying to become pregnant. Advise women of childbearing potential of the need for effective contraception during treatment with siponimod and for 10 days after stopping siponimod. Advise a female patient to immediately inform that prescriber if she is pregnant or planning to become pregnant.
Posterior Reversible Encephalopathy Syndrome
- Advise patients to immediately report to their healthcare provider any symptoms involving sudden onset of severe headache, altered mental status, visual disturbances, or seizure. Inform patients that delayed treatment could lead to permanent neurological sequelae.
Severe Increase in Disability After Stopping Siponimod
- Inform patients that severe increase in disability has been reported after discontinuation of another sphingosine 1-phosphate (S1P) receptor modulator like siponimod. Advise patients to contact their physician if they develop worsening symptoms of MS following discontinuation of siponimod.
Immune System Effects After Stopping Siponimod
- Advise patients that siponimod continues to have effects, such as lowering effects on peripheral lymphocyte count, for up to 3-4 weeks after the last dose.
Storage and Handling
- Instruct patients to store any unopened containers of siponimod in a refrigerator. Inform patients that opened starter packs may be stored at room temperature for 1 week and opened bottles may be stored at room temperature for 1 month.
Medication Guide

Precautions with Alcohol
Alcohol-Siponimod interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Siponimod Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.