Cycloserine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Cycloserine is an antitubercular that is FDA approved for the treatment of active pulmonary and extrapulmonary tuberculosis and in the treatment of acute urinary tract infections caused by susceptible strains of gram positive and gram negative bacteria. Common adverse reactions include confusion, dizziness, headache and somnolence.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Cycloserine is indicated in the treatment of active pulmonary and extrapulmonary tuberculosis (including renal disease) when the causative organisms are susceptible to this drug and when treatment with the primary medications (streptomycin, isoniazid, rifampin, and ethambutol) has proved inadequate.
- Dosage: initial, 250 mg, oral, every 12 h for 2 weeks, then as tolerated to 250 mg every 6 to 8 h; MAX 1 g daily
- Cycloserine may be effective in the treatment of acute urinary tract infections caused by susceptible strains of gram positive and gram negative bacteria, especially Enterobacter spp. and Escherichia coli.
- Dosage: 15 to 20 mg/kg; MAX 1 gram daily
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cycloserine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cycloserine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Cycloserine is indicated in the treatment of active pulmonary and extrapulmonary tuberculosis (including renal disease) when the causative organisms are susceptible to this drug and when treatment with the primary medications (streptomycin, isoniazid, rifampin, and ethambutol) has proved inadequate.
- Dosage: 10 to 20 mg/kg/day ORALLY (divide every 12 h)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cycloserine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cycloserine in pediatric patients.
Contraindications
Administration is contraindicated in patients with any of the following:
- Hypersensitivity to cycloserine
- Epilepsy
- Depression, severe anxiety, or psychosis
- Severe renal insufficiency
- Excessive concurrent use of alcohol
Warnings
- Administration of cycloserine should be discontinued or the dosage reduced if the patient develops allergic dermatitis or symptoms of CNS toxicity, such as convulsions, psychosis, somnolence, depression, confusion, hyperreflexia, headache, tremor, vertigo, paresis, or dysarthria.
- The toxicity of cycloserine is closely related to excessive blood levels (above 30 μg/mL), as determined by high dosage or inadequate renal clearance. The ratio of toxic dose to effective dose in tuberculosis is small.
- The risk of convulsions is increased in chronic alcoholics.
- Administration of cycloserine and other antituberculosis drugs has been associated in a few instances with vitamin B12 and/or folic–acid deficiency, megaloblastic anemia, and sideroblastic anemia.
Adverse Reactions
Clinical Trials Experience
Most adverse reactions occurring during therapy with cycloserine involve the nervous system or are manifestations of drug hypersensitivity. The following side effects have been observed in patients receiving cycloserine:
- Nervous system symptoms (which appear to be related to higher dosages of the drug, i.e., more than 500 mg daily)
- Convulsions
- Drowsiness and somnolence
- Headache
- Tremor
- Dysarthria
- Vertigo
- Confusion and disorientation with loss of memory
- Psychoses, possibly with suicidal tendencies
- Character changes
- Hyperirritability
- Aggression
- Paresis
- Hyperreflexia
- Paresthesia
- Major and minor (localized) clonic seizures
- Coma
- Cardiovascular
- Sudden development of congestive heart failure in patients receiving 1 to 1.5 g of cycloserine daily has been reported
- Allergy (apparently not related to dosage)
- Skin rash
- Miscellaneous
- Elevated serum transaminase, especially in patients with preexisting liver disease.
Postmarketing Experience
There is limited information regarding Cycloserine Postmarketing Experience in the drug label.
Drug Interactions
- Concurrent administration of ethionamide has been reported to potentiate neurotoxic side effects.
- Alcohol and cycloserine are incompatible, especially during a regimen calling for large doses of the latter. Alcohol increases the possibility and risk of epileptic episodes.
- Concurrent administration of isoniazid may result in increased incidence of CNS effects, such as dizziness or drowsiness. Dosage adjustments may be necessary and patients should be monitored closely for signs of CNS toxicity.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
A study in 2 generations of rats given doses up to 100 mg/kg/day demonstrated no teratogenic effect in offspring. It is not known whether cycloserine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Cycloserine should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cycloserine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cycloserine during labor and delivery.
Nursing Mothers
A study in 2 generations of rats given doses up to 100 mg/kg/day demonstrated no teratogenic effect in offspring. It is not known whether cycloserine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Cycloserine should be given to a pregnant woman only if clearly needed.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
There is no FDA guidance on the use of Cycloserine in geriatric settings.
Gender
There is no FDA guidance on the use of Cycloserine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cycloserine with respect to specific racial populations.
Renal Impairment
Blood levels should be determined at least weekly for patients with reduced renal function, for individuals receiving a daily dosage of more than 500 mg, and for those showing signs and symptoms suggestive of toxicity. The dosage should be adjusted to keep the blood level below 30 μg/mL.
Hepatic Impairment
There is no FDA guidance on the use of Cycloserine in patients with hepatic impairment.
Females of Reproductive Potential and Males
- A study in 2 generations of rats showed no impairment of fertility relative to controls for the first mating but somewhat lower fertility in the second mating.
Immunocompromised Patients
There is no FDA guidance one the use of Cycloserine in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
- Doses monitored by blood levels.
- Blood levels should be determined at least weekly for patients with reduced renal function, for individuals receiving a daily dosage of more than 500 mg, and for those showing signs and symptoms suggestive of toxicity.
IV Compatibility
There is limited information regarding the compatibility of Cycloserine and IV administrations.
Overdosage
- Acute toxicity from cycloserine can occur if more than 1 g is ingested by an adult. Chronic toxicity from cycloserine is dose related and can occur if more than 500 mg is administered daily.
- Patients with renal impairment will accumulate cycloserine and may develop toxicity if the dosing regimen is not modified. Patients with severe renal impairment should not receive the drug.
- The central nervous system is the most common organ system involved with toxicity. Toxic effects may include headache, vertigo, confusion, drowsiness, hyperirritability, paresthesias, dysarthria and psychosis.
- Following larger ingestions, paresis, convulsions, and coma often occur.
- Ethyl alcohol may increase the risk of seizures in patients receiving cycloserine.
- The oral median lethal dose in mice is 5290 mg/kg.
Treatment
- To obtain up–to–date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians’ Desk Reference (PDR).
- In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient.
- In adults, many of the neurotoxic effects of cycloserine can be both treated and prevented with the administration of 200 to 300 mg of pyridoxine daily.
- The use of hemodialysis has been shown to remove cycloserine from the bloodstream. This procedure should be reserved for patients with life-threatening toxicity that is unresponsive to less invasive therapy.
Pharmacology
| |
Cycloserine
| |
| Systematic (IUPAC) name | |
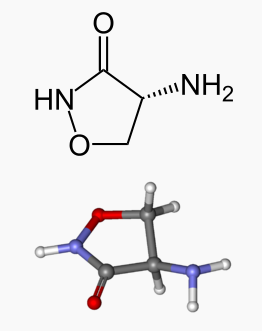
| (R)-4-Amino-1,2-oxazolidin-3-one | |
| Identifiers | |
| CAS number | |
| ATC code | J04 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 102.092 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ~70% to 90% |
| Metabolism | Hepatic |
| Half life | 10 hrs (normal renal function) |
| Excretion | Renal |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
C |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
Cycloserine inhibits cell–wall synthesis in susceptible strains of gram–positive and gram–negative bacteria and in Mycobacterium tuberculosis.
Structure
The molecular weight of cycloserine is 102.09, and it has an empirical formula of C3H6N2O2.
Pharmacodynamics
There is limited information regarding Cycloserine Pharmacodynamics in the drug label.
Pharmacokinetics
After oral administration, cycloserine is readily absorbed from the gastrointestinal tract, with peak blood levels occurring in 4 to 8 hours. Blood levels of 25 to 30 μg/mL can generally be maintained with the usual dosage of 250 mg twice a day, although the relationship of plasma levels to dosage is not always consistent. Concentrations in the cerebrospinal fluid, pleural fluid, fetal blood, and mother’s milk approach those found in the serum. Detectable amounts are found in ascitic fluid, bile, sputum, amniotic fluid, and lung and lymph tissues. Approximately 65 percent of a single dose of cycloserine can be recovered in the urine within 72 hours after oral administration. The remaining 35 percent is apparently metabolized to unknown substances. The maximum excretion rate occurs 2 to 6 hours after administration, with 50 percent of the drug eliminated in 12 hours.
Nonclinical Toxicology
Carcinogenesis and Mutagenicity
- Studies have not been performed to determine potential for carcinogenicity.
- The Ames test and unscheduled DNA repair test were negative.
Clinical Studies
There is limited information regarding Cycloserine Clinical Studies in the drug label.
How Supplied
Cycloserine is available as a 250 mg capsule
- Bottles of 40 capsules
- NDC 13845-1201-3
Storage
Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Cycloserine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Cycloserine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Cycloserine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Cycloserine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Seromycin [1]
Look-Alike Drug Names
There is limited information regarding Cycloserine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Cycloserine |Label Name=Cycloserine 250 mg.png
}}