Sargramostim
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Sargramostim is a colony stimulating factor that is FDA approved for the treatment of granulocytopenia following induction chemotherapy in acute myelogenous leukemia, mobilization and following transplantation of autologous peripheral blood progentior cells, myeloid reconstitution after autologous bone marrow transplantation, myeloid reconstitution after allogeneic bone marrow transplantation and bone marrow transplantation failure or engraftment delay. Common adverse reactions include chest pain, peripheral edema, pruritus, rash, hypercholesterolemia, hypomagnesemia, weight loss, abdominal pain, diarrhea, dysphagia, GI hemorrhage, hematemesis, nausea, vomiting, acute myelogenous leukemia, increased bilirrubin, arthralgia, bone pain, myalgia, asthenia, intraocular hemorrhage, anxiety, elevated BUN, pharyngitis, fever, malaise and rigor.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Neutrophil Recovery Following Chemotherapy in Acute Myelogenous Leukemia
- The recommended dose is 250 mcg/m2/day administered intravenously over a 4 hour period starting approximately on day 11 or four days following the completion of induction chemotherapy, if the day 10 bone marrow is hypoplastic with <5% blasts. If a second cycle of induction chemotherapy is necessary, Sargramostim should be administered approximately four days after the completion of chemotherapy if the bone marrow is hypoplastic with <5% blasts. Sargramostim should be continued until an ANC >1500 cells/mm3 for 3 consecutive days or a maximum of 42 days. Sargramostim should be discontinued immediately if leukemic regrowth occurs. If a severe adverse reaction occurs, the dose can be reduced by 50% or temporarily discontinued until the reaction abates.
- In order to avoid potential complications of excessive leukocytosis (WBC > 50,000 cells/mm3 or ANC > 20,000 cells/mm3) a CBC with differential is recommended twice per week during Sargramostim therapy. Sargramostim treatment should be interrupted or the dose reduced by half if the ANC exceeds 20,000 cells/mm3.
Mobilization of Peripheral Blood Progenitor Cells
- The recommended dose is 250 mcg/m2/day administered IV over 24 hours or SC once daily. Dosing should continue at the same dose through the period of PBPC collection. The optimal schedule for PBPC collection has not been established. In clinical studies, collection of PBPC was usually begun by day 5 and performed daily until protocol specified targets were achieved. If WBC > 50,000 cells/mm3, the Sargramostim dose should be reduced by 50%. If adequate numbers of progenitor cells are not collected, other mobilization therapy should be considered.
Post Peripheral Blood Progenitor Cell Transplantation
- The recommended dose is 250 mcg/m2/day administered IV over 24 hours or SC once daily beginning immediately following infusion of progenitor cells and continuing until an ANC>1500 cells/mm3 for three consecutive days is attained.
Myeloid Reconstitution After Autologous or Allogeneic Bone Marrow Transplantation
- The recommended dose is 250 mcg/m2/day administered IV over a 2-hour period beginning two to four hours after bone marrow infusion, and not less than 24 hours after the last dose of chemotherapy or radiotherapy. Patients should not receive Sargramostim until the post marrow infusion ANC is less than 500 cells/mm3. Sargramostim should be continued until an ANC >1500 cells/mm3 for three consecutive days is attained. If a severe adverse reaction occurs, the dose can be reduced by 50% or temporarily discontinued until the reaction abates. Sargramostim should be discontinued immediately if blast cells appear or disease progression occurs.
- In order to avoid potential complications of excessive leukocytosis (WBC > 50,000 cells/mm3, ANC > 20,000 cells/mm3) a CBC with differential is recommended twice per week during Sargramostim therapy. Sargramostim treatment should be interrupted or the dose reduced by 50% if the ANC exceeds 20,000 cells/mm3.
Bone Marrow Transplantation Failure or Engraftment Delay
- The recommended dose is 250 mcg/m2/day for 14 days as a 2-hour IV infusion. The dose can be repeated after 7 days off therapy if engraftment has not occurred. If engraftment still has not occurred, a third course of 500 mcg/m2/day for 14 days may be tried after another 7 days off therapy. If there is still no improvement, it is unlikely that further dose escalation will be beneficial. If a severe adverse reaction occurs, the dose can be reduced by 50% or temporarily discontinued until the reaction abates. Sargramostim should be discontinued immediately if blast cells appear or disease progression occurs.
- In order to avoid potential complications of excessive leukocytosis (WBC > 50,000 cells/mm3, ANC > 20,000 cells/mm3) a CBC with differential is recommended twice per week during Sargramostim therapy. Sargramostim treatment should be interrupted or the dose reduced by half if the ANC exceeds 20,000 cells/mm3.
Preparation of Sargramostim
- Liquid Sargramostim is formulated as a sterile, preserved (1.1% benzyl alcohol), injectable solution (500 mcg/mL) in a vial. Lyophilized Sargramostim is a sterile, white, preservative-free powder (250 mcg) that requires reconstitution with 1 mL Sterile Water for Injection, USP, or 1 mL Bacteriostatic Water for Injection, USP.
- Liquid Sargramostim may be stored for up to 20 days at 2–8°C once the vial has been entered. Discard any remaining solution after 20 days.
- Lyophilized Sargramostim (250 mcg) should be reconstituted aseptically with 1.0 mL of diluent (see below).
- The contents of vials reconstituted with different diluents should not be mixed together. Sterile Water for Injection, USP (without preservative): Lyophilized Sargramostim vials contain no antibacterial preservative, and therefore solutions prepared with Sterile Water for Injection, USP should be administered as soon as possible, and within 6 hours following reconstitution and/or dilution for IV infusion. The vial should not be re-entered or reused. Do not save any unused portion for administration more than 6 hours following reconstitution. Bacteriostatic Water for Injection, USP (0.9% benzyl alcohol): Reconstituted solutions prepared with Bacteriostatic Water for Injection, USP (0.9% benzyl alcohol) may be stored for up to 20 days at 2–8°C prior to use. Discard reconstituted solution after 20 days. Previously reconstituted solutions mixed with freshly reconstituted solutions must be administered within 6 hours following mixing. Preparations containing benzyl alcohol (including liquid Sargramostim and lyophilized Sargramostim reconstituted with Bacteriostatic Water for Injection) should not be used in neonates.
- During reconstitution of lyophilized Sargramostim the diluent should be directed at the side of the vial and the contents gently swirled to avoid foaming during dissolution. Avoid excessive or vigorous agitation; do not shake.
- Sargramostim should be used for SC injection without further dilution. Dilution for IV infusion should be performed in 0.9% Sodium Chloride Injection, USP. If the final concentration of Sargramostim is below 10 mcg/mL, Albumin (Human) at a final concentration of 0.1% should be added to the saline prior to addition of Sargramostim to prevent adsorption to the components of the drug delivery system. To obtain a final concentration of 0.1% Albumin (Human), add 1 mg Albumin (Human) per 1 mL 0.9% Sodium Chloride Injection, USP (e.g., use 1 mL 5% Albumin [Human] in 50 mL 0.9% Sodium Chloride Injection, USP).
An in-line membrane filter should NOT be used for intravenous infusion of Sargramostim
- Store liquid Sargramostim and reconstituted lyophilized Sargramostim solutions under refrigeration at 2–8°C (36–46°F); DO NOT FREEZE.
- In the absence of compatibility and stability information, no other medication should be added to infusion solutions containing Sargramostim. Use only 0.9% Sodium Chloride Injection, USP to prepare IV infusion solutions.
- Aseptic technique should be employed in the preparation of all Sargramostim solutions. To assure correct concentration following reconstitution, care should be exercised to eliminate any air bubbles from the needle hub of the syringe used to prepare the diluent. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. If particulate matter is present or the solution is discolored, the vial should not be used.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sargramostim in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sargramostim in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Sargramostim FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sargramostim in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sargramostim in pediatric patients.
Contraindications
Sargramostim is contraindicated:
- In patients with excessive leukemic myeloid blasts in the bone marrow or peripheral blood (≥ 10%);
- In patients with known hypersensitivity to GM-CSF, yeast-derived products or any component of the product;
- For concomitant use with chemotherapy and radiotherapy.
- Due to the potential sensitivity of rapidly dividing hematopoietic progenitor cells, Sargramostim should not be administered simultaneously with cytotoxic chemotherapy or radiotherapy or within 24 hours preceding or following chemotherapy or radiotherapy. In one controlled study, patients with small cell lung cancer received Sargramostim and concurrent thoracic radiotherapy and chemotherapy or the identical radiotherapy and chemotherapy without Sargramostim The patients randomized to Sargramostim had significantly higher incidence of adverse events, including higher mortality and a higher incidence of grade 3 and 4 infections and grade 3 and 4 thrombocytopenia.
Warnings
Pediatric Use
- Benzyl alcohol is a constituent of liquid Sargramostim and Bacteriostatic Water for Injection diluent. Benzyl alcohol has been reported to be associated with a fatal "Gasping Syndrome" in premature infants. Liquid solutions containing benzyl alcohol (including liquid Sargramostim) or lyophilized Sargramostim reconstituted with Bacteriostatic Water for Injection, USP (0.9% benzyl alcohol) should not be administered to neonates.
Fluid Retention
- Edema, capillary leak syndrome, pleural effusion and/or pericardial effusion have been reported in patients after Sargramostim administration. In 156 patients enrolled in placebo-controlled studies using Sargramostim at a dose of 250 mcg/m2/day by 2-hour IV infusion, the reported incidences of fluid retention (Sargramostim vs. placebo) were as follows: peripheral edema, 11% vs. 7%; pleural effusion, 1% vs. 0%; and pericardial effusion, 4% vs. 1%. Capillary leak syndrome was not observed in this limited number of studies; based on other uncontrolled studies and reports from users of marketed Sargramostim, the incidence is estimated to be less than 1%. In patients with preexisting pleural and pericardial effusions, administration of Sargramostim may aggravate fluid retention; however, fluid retention associated with or worsened by Sargramostim has been reversible after interruption or dose reduction of Sargramostim with or without diuretic therapy. Sargramostim should be used with caution in patients with preexisting fluid retention, pulmonary infiltrates or congestive heart failure.
Respiratory Symptoms
- Sequestration of granulocytes in the pulmonary circulation has been documented following Sargramostim infusion and dyspnea has been reported occasionally in patients treated with Sargramostim Special attention should be given to respiratory symptoms during or immediately following Sargramostim infusion, especially in patients with preexisting lung disease. In patients displaying dyspnea during Sargramostim administration, the rate of infusion should be reduced by half. If respiratory symptoms worsen despite infusion rate reduction, the infusion should be discontinued. Subsequent IV infusions may be administered following the standard dose schedule with careful monitoring. Sargramostim should be administered with caution in patients with hypoxia.
Cardiovascular Symptoms
- Occasional transient supraventricular arrhythmia has been reported in uncontrolled studies during Sargramostim administration, particularly in patients with a previous history of cardiac arrhythmia. However, these arrhythmias have been reversible after discontinuation of Sargramostim. Sargramostim should be used with caution in patients with preexisting cardiac disease.
Renal and Hepatic Dysfunction
- In some patients with preexisting renal dysfunction or hepatic dysfunction enrolled in uncontrolled clinical trials, administration of Sargramostim has induced elevation of serum creatinine or bilirubin and hepatic enzymes. Dose reduction or interruption of Sargramostim administration has resulted in a decrease to pretreatment values. However, in controlled clinical trials the incidences of renal dysfunction and hepatic dysfunction were comparable between Sargramostim (250 mcg/m2/day by 2-hour IV infusion) and placebo-treated patients. Monitoring of renal function and hepatic function in patients displaying renal or hepatic dysfunction prior to initiation of treatment is recommended at least every other week during Sargramostim administration.
Adverse Reactions
Clinical Trials Experience
Autologous and Allogeneic Bone Marrow Transplantation
- Sargramostim is generally well tolerated. In three placebo-controlled studies enrolling a total of 156 patients after autologous BMT or peripheral blood progenitor cell transplantation, events reported in at least 10% of patients who received IV Sargramostim or placebo were as reported in TABLE 6.

- No significant differences were observed between Sargramostim and placebo-treated patients in the type or frequency of laboratory abnormalities, including renal and hepatic parameters. In some patients with preexisting renal or hepatic dysfunction enrolled in uncontrolled clinical trials, administration of Sargramostim has induced elevation of serum creatinine or bilirubin and hepatic enzymes (see WARNINGS). In addition, there was no significant difference in relapse rate and 24 month survival between the Sargramostim and placebo-treated patients.
- In the placebo-controlled trial of 109 patients after allogeneic BMT, events reported in at least 10% of patients who received IV Sargramostim or placebo were as reported in TABLE 7.

- There were no significant differences in the incidence or severity of GVHD, relapse rates and survival between the Sargramostim and placebo-treated patients. Adverse events observed for the patients treated with Sargramostim in the historically-controlled BMT failure study were similar to those reported in the placebo-controlled studies. In addition, headache (26%), pericardial effusion (25%), arthralgia (21%) and myalgia (18%) were also reported in patients treated with Sargramostim in the graft failure study.
- In uncontrolled Phase I/II studies with Sargramostim in 215 patients, the most frequent adverse events were fever, asthenia, headache, bone pain, chills and myalgia. These systemic events were generally mild or moderate and were usually prevented or reversed by the administration of analgesics and antipyretics such as acetaminophen. In these uncontrolled trials, other infrequent events reported were dyspnea, peripheral edema, and rash.
- Reports of events occurring with marketed Sargramostim include arrhythmia, fainting, eosinophilia, dizziness, hypotension, injection site reactions, pain (including abdominal, back, chest, and joint pain), tachycardia, thrombosis, and transient liver function abnormalities.
- In patients with preexisting edema, capillary leak syndrome, pleural effusion and/or pericardial effusion, administration of Sargramostim may aggravate fluid retention. Body weight and hydration status should be carefully monitored during Sargramostim administration.
- Adverse events observed in pediatric patients in controlled studies were comparable to those observed in adult patients.
Acute Myelogenous Leukemia
Adverse events reported in at least 10% of patients who received Sargramostim or placebo were as reported in TABLE 8.

- Nearly all patients reported leukopenia, thrombocytopenia and anemia. The frequency and type of adverse events observed following induction were similar between Sargramostim and placebo groups. The only significant difference in the rates of these adverse events was an increase in skin associated events in the Sargramostim group (p=0.002). No significant differences were observed in laboratory results, renal or hepatic toxicity. No significant differences were observed between the Sargramostim and placebo-treated patients for adverse events following consolidation. There was no significant difference in response rate or relapse rate.
- In a historically-controlled study of 86 patients with acute myelogenous leukemia (AML), the Sargramostim treated group exhibited an increased incidence of weight gain (p=0.007), low serum proteins and prolonged prothrombin time (p=0.02) when compared to the control group. Two Sargramostim treated patients had progressive increase in circulating monocytes and promonocytes and blasts in the marrow which reversed when Sargramostim was discontinued. The historical control group exhibited an increased incidence of cardiac events (p=0.018), liver function abnormalities (p=0.008), and neurocortical hemorrhagic events (p=0.025).15
Antibody Formation
- Serum samples collected before and after Sargramostim treatment from 214 patients with a variety of underlying diseases have been examined for immunogenicity based on the presence of antibodies. Neutralizing antibodies were detected in five of 214 patients (2.3%) after receiving Sargramostim by continuous IV infusion (three patients) or subcutaneous injection (SC) (two patients) for 28 to 84 days in multiple courses. All five patients had impaired hematopoiesis before the administration of Sargramostim and consequently the effect of the development of anti-GM-CSF antibodies on normal hematopoiesis could not be assessed. Antibody studies of 75 patients with Crohn's disease receiving Sargramostim by subcutaneous injection with normal hematopoiesis and no other immunosuppressive drugs showed one patient (1.3%) with detectable neutralizing antibodies. The clinical relevance of the presence of these antibodies are unknown. Drug-induced neutropenia, neutralization of endogenous GM-CSF activity and diminution of the therapeutic effect of Sargramostim secondary to formation of neutralizing antibody remain a theoretical possibility. Serious allergic and anaphylactoid reactions have been reported with Sargramostim but the rate of occurrence of antibodies in such patients has not been assessed.
Overdosage
- The maximum amount of Sargramostim that can be safely administered in single or multiple doses has not been determined. Doses up to 100 mcg/kg/day (4,000 mcg/m2/day or 16 times the recommended dose) were administered to four patients in a Phase I uncontrolled clinical study by continuous IV infusion for 7 to 18 days. Increases in WBC up to 200,000 cells/mm3 were observed. Adverse events reported were dyspnea, malaise, nausea, fever, rash, sinus tachycardia, headache and chills. All these events were reversible after discontinuation of Sargramostim.
- In case of overdosage, Sargramostim therapy should be discontinued and the patient carefully monitored for WBC increase and respiratory symptoms.
To report SUSPECTED ADVERSE REACTIONS, contact Genzyme Corporation at 1-888-4RX-Sargramostim or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Postmarketing Experience
There is limited information regarding Sargramostim Postmarketing Experience in the drug label.
Drug Interactions
- Interactions between Sargramostim and other drugs have not been fully evaluated. Drugs which may potentiate the myeloproliferative effects of Sargramostim, such as lithium and corticosteroids, should be used with caution.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Animal reproduction studies have not been conducted with Sargramostim It is not known whether Sargramostim can cause fetal harm when administered to a pregnant woman or can affect reproductive capability. Sargramostim should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sargramostim in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sargramostim during labor and delivery.
Nursing Mothers
- It is not known whether Sargramostim is excreted in human milk. Because many drugs are excreted in human milk, Sargramostim should be administered to a nursing woman only if clearly needed.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established; however, available safety data indicate that Sargramostim does not exhibit any greater toxicity in pediatric patients than in adults. A total of 124 pediatric subjects between the ages of 4 months and 18 years have been treated with Sargramostim in clinical trials at doses ranging from 60–1,000 mcg/m2/day intravenously and 4–1,500 mcg/m2/day subcutaneously. In 53 pediatric patients enrolled in controlled studies at a dose of 250 mcg/m2/day by 2-hour IV infusion, the type and frequency of adverse events were comparable to those reported for the adult population. Liquid solutions containing benzyl alcohol (including liquid Sargramostim or lyophilized Sargramostim reconstituted with Bacteriostatic Water for Injection, USP (0.9% benzyl alcohol) should not be administered to neonates
Geriatic Use
- In the clinical trials, experience in older patients (age ≥65 years), was limited to the acute myelogenous leukemia (AML) study. Of the 52 patients treated with Sargramostim in this randomized study, 22 patients were age 65–70 years and 30 patients were age 55–64 years. The number of placebo patients in each age group were 13 and 33 patients respectively. This was not an adequate database from which determination of differences in efficacy endpoints or safety assessments could be reliably made and this clinical study was not designed to evaluate difference between these two age groups. Analyses of general trends in safety and efficacy were undertaken and demonstrate similar patterns for older (65–70 yrs) vs younger patients (55–64 yrs). Greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Sargramostim with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sargramostim with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sargramostim in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sargramostim in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sargramostim in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sargramostim in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Sargramostim Administration in the drug label.
Monitoring
There is limited information regarding Sargramostim Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Sargramostim and IV administrations.
Overdosage
- The maximum amount of Sargramostim that can be safely administered in single or multiple doses has not been determined. Doses up to 100 mcg/kg/day (4,000 mcg/m2/day or 16 times the recommended dose) were administered to four patients in a Phase I uncontrolled clinical study by continuous IV infusion for 7 to 18 days. Increases in WBC up to 200,000 cells/mm3 were observed. Adverse events reported were dyspnea, malaise, nausea, fever, rash, sinus tachycardia, headache and chills. All these events were reversible after discontinuation of Sargramostim.
- In case of overdosage, Sargramostim therapy should be discontinued and the patient carefully monitored for WBC increase and respiratory symptoms.
Pharmacology
Sargramostim
| |
| Systematic (IUPAC) name | |
| Human granulocyte macrophage colony stimulating factor | |
| Identifiers | |
| CAS number | |
| ATC code | L03 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 14434.5 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Rx only |
| Routes | ? |
Mechanism of Action
There is limited information regarding Sargramostim Mechanism of Action in the drug label.
Structure
There is limited information regarding Sargramostim Structure in the drug label.
Pharmacodynamics
In vitro Studies of Sargramostim in Human Cells
- The biological activity of GM-CSF is species-specific. Consequently, in vitro studies have been performed on human cells to characterize the pharmacological activity of Sargramostim. In vitro exposure of human bone marrow cells to Sargramostim at concentrations ranging from 1–100 ng/mL results in the proliferation of hematopoietic progenitors and in the formation of pure granulocyte, pure macrophage and mixed granulocytemacrophage colonies. Chemotactic, anti-fungal and anti-parasitic activities of granulocytes and monocytes are increased by exposure to Sargramostim in vitro. Sargramostim increases the cytotoxicity of monocytes toward certain neoplastic cell lines and activates polymorphonuclear neutrophils to inhibit the growth of tumor cells.
Pharmacokinetics
General
- GM-CSF belongs to a group of growth factors termed colony stimulating factors which support survival, clonal expansion, and differentiation of hematopoietic progenitor cells. GM-CSF induces partially committed progenitor cells to divide and differentiate in the granulocyte-macrophage pathways which include neutrophils, monocytes/macrophages and myeloid-derived dendritic cells.
- GM-CSF is also capable of activating mature granulocytes and macrophages. GM-CSF is a multilineage factor and, in addition to dose-dependent effects on the myelomonocytic lineage, can promote the proliferation of megakaryocytic and erythroid progenitors. However, other factors are required to induce complete maturation in these two lineages. The various cellular responses (i.e., division, maturation, activation) are induced through GM-CSF binding to specific receptors expressed on the cell surface of target cells.2
In vivo Primate Studies of Sargramostim
- Pharmacology/toxicology studies of Sargramostim were performed in cynomolgus monkeys. An acute toxicity study revealed an absence of treatment-related toxicity following a single IV bolus injection at a dose of 300 mcg/kg. Two subacute studies were performed using IV injection (maximum dose 200 mcg/kg/day × 14 days) and subcutaneous injection (SC) (maximum dose 200 mcg/kg/day × 28 days). No major visceral organ toxicity was documented. Notable histopathology findings included increased cellularity in hematologic organs and heart and lung tissues. A dose-dependent increase in leukocyte count, which consisted primarily of segmented neutrophils, occurred during the dosing period; increases in monocytes, basophils, eosinophils and lymphocytes were also noted. Leukocyte counts decreased to pretreatment values over a 1–2 week recovery period.
Pharmacokinetics
- Pharmacokinetic profiles have been analyzed in controlled studies of 24 normal male volunteers. Liquid and lyophilized Sargramostim at the recommended dose of 250 mcg/m2, have been determined to be bioequivalent based on the statistical evaluation of AUC.5
- When Sargramostim (either liquid or lyophilized) was administered IV over two hours to normal volunteers, the mean beta half-life was approximately 60 minutes. Peak concentrations of GM-CSF were observed in blood samples obtained during or immediately after completion of Sargramostim infusion. For liquid Sargramostim the mean maximum concentration (Cmax) was 5.0 ng/mL, the mean clearance rate was approximately 420 mL/min/m2 and the mean AUC (0–inf) was 640 ng/mL•min. Corresponding results for lyophilized Sargramostim in the same subjects were mean Cmax of 5.4 ng/mL, mean clearance rate of 431 mL/min/m2, and mean AUC (0–inf) of 677 ng/mL•min. GM-CSF was last detected in blood samples obtained at three or six hours.
- When Sargramostim (either liquid or lyophilized) was administered SC to normal volunteers, GM-CSF was detected in the serum at 15 minutes, the first sample point. The mean beta half-life was approximately 162 minutes. Peak levels occurred at one to three hours post injection, and Sargramostim remained detectable for up to six hours after injection. The mean Cmax was 1.5 ng/mL. For liquid Sargramostim the mean clearance was 549 mL/min/m2 and the mean AUC (0–inf) was 549 ng/mL•min. For lyophilized Sargramostim, the mean clearance was 529 mL/min/m2 and the mean AUC (0–inf) was 501 ng/mL•min
Nonclinical Toxicology
There is limited information regarding Sargramostim Nonclinical Toxicology in the drug label.
Clinical Studies
Acute Myelogenous Leukemia
- The safety and efficacy of Sargramostim in patients with AML who are younger than 55 years of age have not been determined. Based on Phase II data suggesting the best therapeutic effects could be achieved in patients at highest risk for severe infections and mortality while neutropenic, the Phase III clinical trial was conducted in older patients. The safety and efficacy of Sargramostim in the treatment of AML were evaluated in a multi-center, randomized, double-blind placebo-controlled trial of 99 newly diagnosed adult patients, 55–70 years of age, receiving induction with or without consolidation. A combination of standard doses of daunorubicin (days 1–3) and ara-C (days 1–7) was administered during induction and high dose ara-C was administered days 1–6 as a single course of consolidation, if given. Bone marrow evaluation was performed on day 10 following induction chemotherapy. If hypoplasia with <5% blasts was not achieved, patients immediately received a second cycle of induction chemotherapy. If the bone marrow was hypoplastic with <5% blasts on day 10 or four days following the second cycle of induction chemotherapy, Sargramostim (250 mcg/m2/day) or placebo was given IV over four hours each day, starting four days after the completion of chemotherapy. Study drug was continued until an ANC ≥1500/mm3 for three consecutive days was attained or a maximum of 42 days. Sargramostim or placebo was also administered after the single course of consolidation chemotherapy if delivered (ara-C 3–6 weeks after induction following neutrophil recovery). Study drug was discontinued immediately if leukemic regrowth occurred.
- Sargramostim significantly shortened the median duration of ANC <500/mm3 by 4 days and <1000/mm3 by 7 days following induction (see TABLE 1). 75% of patients receiving Sargramostim achieved ANC >500/mm3 by day 16, compared to day 25 for patients receiving placebo. The proportion of patients receiving one cycle (70%) or two cycles (30%) of induction was similar in both treatment groups; Sargramostim significantly shortened the median times to neutrophil recovery whether one cycle (12 versus 15 days) or two cycles (14 versus 23 days) of induction chemotherapy was administered. Median times to platelet (>20,000/mm3) and RBC transfusion independence were not significantly different between treatment groups.

- During the consolidation phase of treatment, Sargramostim did not shorten the median time to recovery of ANC to 500/mm3 (13 days) or 1000/mm3 (14.5 days) compared to placebo. There were no significant differences in time to platelet and RBC transfusion independence.
- The incidence of severe infections and deaths associated with infections was significantly reduced in patients who received Sargramostim During induction or consolidation, 27 of 52 patients receiving Sargramostim and 35 of 47 patients receiving placebo had at least one grade 3, 4 or 5 infection (p=0.02). Twenty-five patients receiving Sargramostim and 30 patients receiving placebo experienced severe and fatal infections during induction only. There were significantly fewer deaths from infectious causes in the Sargramostim arm (3 versus 11, p=0.02). The majority of deaths in the placebo group were associated with fungal infections with pneumonia as the primary infection.
- Disease outcomes were not adversely affected by the use of Sargramostim The proportion of patients achieving complete remission (CR) was higher in the Sargramostim group (69% as compared to 55% for the placebo group), but the difference was not significant (p=0.21). There was no significant difference in relapse rates; 12 of 36 patients who received Sargramostim and five of 26 patients who received placebo relapsed within 180 days of documented CR (p=0.26). The overall median survival was 378 days for patients receiving Sargramostim and 268 days for those on placebo (p=0.17). The study was not sized to assess the impact of Sargramostim treatment on response or survival.
Mobilization and Engraftment of PBPC
- A retrospective review was conducted of data from patients with cancer undergoing collection of peripheral blood progenitor cells (PBPC) at a single transplant center. Mobilization of PBPC and myeloid reconstitution post-transplant were compared between four groups of patients (n=196) receiving Sargramostim for mobilization and a historical control group who did not receive any mobilization treatment (progenitor cells collected by leukapheresis without mobilization (n=100)). Sequential cohorts received Sargramostim The cohorts differed by dose (125 or 250 mcg/m2/day), route (IV over 24 hours or SC) and use of Sargramostim post-transplant. Leukaphereses were initiated for all mobilization groups after the WBC reached 10,000/mm3. Leukaphereses continued until both a minimum number of mononucleated cells (MNC) were collected (6.5 or 8.0 × 108/kg body weight) and a minimum number of phereses (5–8) were performed. Both minimum requirements varied by treatment cohort and planned conditioning regimen. If subjects failed to reach a WBC of 10,000 cells/mm3 by day five, another cytokine was substituted for Sargramostim; these subjects were all successfully leukapheresed and transplanted. The most marked mobilization and post-transplant effects were seen in patients administered the higher dose of Sargramostim (250 mcg/m2) either IV (n=63) or SC (n=41).
- PBPCs from patients treated at the 250 mcg/m2/day dose had significantly higher number of granulocyte-macrophage colony-forming units (CFU-GM) than those collected without mobilization. The mean value after thawing was 11.41 × 104 CFU-GM/kg for all Sargramostim-mobilized patients, compared to 0.96 × 104/kg for the non-mobilized group. A similar difference was observed in the mean number of erythrocyte burst-forming units (BFU-E) collected (23.96 × 104/kg for patients mobilized with 250 mcg/m2 doses of Sargramostim administered SC vs. 1.63 × 104/kg for non-mobilized patients).
- After transplantation, mobilized subjects had shorter times to myeloid engraftment and fewer days between transplantation and the last platelet transfusion compared to non-mobilized subjects. Neutrophil recovery (ANC >500/mm3) was more rapid in patients administered Sargramostim following PBPC transplantation with Sargramostim-mobilized cells (see TABLE 2). Mobilized patients also had fewer days to the last platelet transfusion and last RBC transfusion, and a shorter duration of hospitalization than did non-mobilized subjects.

- A second retrospective review of data from patients undergoing PBPC at another single transplant center was also conducted. Sargramostim was given SC at 250 mcg/m2/day once a day (n=10) or twice a day (n=21) until completion of the phereses. Phereses were begun on day 5 of Sargramostim administration and continued until the targeted MNC count of 9 × 108/kg or CD34+ cell count of 1 × 106/kg was reached. There was no difference in CD34+ cell count in patients receiving Sargramostim once or twice a day. The median time to ANC>500/mm3 was 12 days and to platelet recovery (>25,000/mm3) was 23 days.
- Survival studies comparing mobilized study patients to the nonmobilized patients and to an autologous historical bone marrow transplant group showed no differences in median survival time.
Autologous Bone Marrow Transplantation
Following a dose-ranging Phase I/II trial in patients undergoing autologous BMT for lymphoid malignancies, three single center, randomized, placebo-controlled and double-blinded studies were conducted to evaluate the safety and efficacy of Sargramostim for promoting hematopoietic reconstitution following autologous BMT. A total of 128 patients (65 Sargramostim 63 placebo) were enrolled in these three studies. The majority of the patients had lymphoid malignancy (87 NHL, 17 ALL), 23 patients had Hodgkin's disease, and one patient had acute myeloblastic leukemia (AML). In 72 patients with NHL or ALL, the bone marrow harvest was purged prior to storage with one of several monoclonal antibodies. No chemical agent was used for in vitro treatment of the bone marrow. Preparative regimens in the three studies included cyclophosphamide (total dose 120–150 mg/kg) and total body irradiation (total dose 1,200–1,575 rads). Other regimens used in patients with Hodgkin's disease and NHL without radiotherapy consisted of three or more of the following in combination (expressed as total dose): cytosine arabinoside (400 mg/m2) and carmustine (300 mg/m2), cyclophosphamide (140–150 mg/kg), hydroxyurea (4.5 grams/m2) and etoposide (375–450 mg/m2).
- Compared to placebo, administration of Sargramostim in two studies (n=44 and 47) significantly improved the following hematologic and clinical endpoints: time to neutrophil engraftment, duration of hospitalization and infection experience or antibacterial usage. In the third study (n=37) there was a positive trend toward earlier myeloid engraftment in favor of Sargramostim This latter study differed from the other two in having enrolled a large number of patients with Hodgkin's disease who had also received extensive radiation and chemotherapy prior to harvest of autologous bone marrow. A subgroup analysis of the data from all three studies revealed that the median time to engraftment for patients with Hodgkin's disease, regardless of treatment, was six days longer when compared to patients with NHL and ALL, but that the overall beneficial Sargramostim treatment effect was the same. In the following combined analysis of the three studies, these two subgroups (NHL and ALL vs. Hodgkin's disease) are presented separately.

Patients with Lymphoid Malignancy (Non-Hodgkin's Lymphoma and Acute Lymphoblastic Leukemia)
- Myeloid engraftment (absolute neutrophil count ANC≥500 cells/mm3) in 54 patients receiving Sargramostim was observed 6 days earlier than in 50 patients treated with placebo (see TABLE 3). Accelerated myeloid engraftment was associated with significant clinical benefits. The median duration of hospitalization was six days shorter for the Sargramostim group than for the placebo group. Median duration of infectious episodes (defined as fever and neutropenia; or two positive cultures of the same organism; or fever >38°C and one positive blood culture; or clinical evidence of infection) was three days less in the group treated with Sargramostim. The median duration of antibacterial administration in the post-transplantation period was four days shorter for the patients treated with Sargramostim than for placebo-treated patients. The study was unable to detect a significant difference between the treatment groups in rate of disease relapse 24 months post-transplantation. As a group, leukemic subjects receiving Sargramostim derived less benefit than NHL subjects. However, both the leukemic and NHL groups receiving Sargramostim engrafted earlier than controls.
Patients with Hodgkin's Disease
- If patients with Hodgkin's disease are analyzed separately, a trend toward earlier myeloid engraftment is noted. Sargramostim-treated patients engrafted earlier (by five days) than the placebo-treated patients (p=0.189, Wilcoxon) but the number of patients was small (n=22).
Allogeneic Bone Marrow Transplantation
- A multi-center, randomized, placebo-controlled, and double-blinded study was conducted to evaluate the safety and efficacy of Sargramostim for promoting hematopoietic reconstitution following allogeneic BMT. A total of 109 patients (53 Sargramostim, 56 placebo) were enrolled in the study. Twenty-three patients (11 Sargramostim, 12 placebo) were 18 years old or younger. Sixty-seven patients had myeloid malignancies (33 AML, 34 CML), 17 had lymphoid malignancies (12 ALL, 5 NHL), three patients had Hodgkin's disease, six had multiple myeloma, nine had myelodysplastic disease, and seven patients had aplastic anemia. In 22 patients at one of the seven study sites, bone marrow harvests were depleted of T cells. Preparative regimens included cyclophosphamide, busulfan, cytosine arabinoside, etoposide, methotrexate, corticosteroids, and asparaginase. Some patients also received total body, splenic, or testicular irradiation. Primary graft-versus-host disease (GVHD) prophylaxis was cyclosporine A and a corticosteroid.
- Accelerated myeloid engraftment was associated with significant laboratory and clinical benefits. Compared to placebo, administration of Sargramostim significantly improved the following: time to neutrophil engraftment, duration of hospitalization, number of patients with bacteremia and overall incidence of infection (see TABLE 4).

- Median time to myeloid engraftment (ANC ≥ 500 cells/mm3) in 53 patients receiving Sargramostim was 4 four days less than in 56 patients treated with placebo (see TABLE 4). The number of patients with bacteremia and infection was significantly lower in the Sargramostim group compared to the placebo group (9/53 versus 19/56 and 30/53 versus 42/56, respectively). There were a number of secondary laboratory and clinical endpoints. Of these, only the incidence of severe (grade 3/4) mucositis was significantly improved in the Sargramostim group (4/53) compared to the placebo group (16/56) at p<0.05. Sargramostim-treated patients also had a shorter median duration of post-transplant IV antibiotic infusions, and shorter median number of days to last platelet and RBC transfusions compared to placebo patients, but none of these differences reached statistical significance.
Bone Marrow Transplantation Failure or Engraftment Delay
- A historically-controlled study was conducted in patients experiencing graft failure following allogeneic or autologous BMT to determine whether Sargramostim improved survival after BMT failure.
- Three categories of patients were eligible for this study:
- patients displaying a delay in engraftment (ANC ≤ 100 cells/mm3 by day 28 post-transplantation);
- patients displaying a delay in engraftment (ANC ≤ 100 cells/mm3 by day 21 post-transplantation) and who had evidence of an active infection; and
- patients who lost their marrow graft after a transient engraftment (manifested by an average of ANC ≥ 500 cells/mm3 for at least one week followed by loss of engraftment with ANC < 500 cells/mm3 for at least one week beyond day 21 post-transplantation).
- A total of 140 eligible patients from 35 institutions were treated with Sargramostim and evaluated in comparison to 103 historical control patients from a single institution. One hundred sixty-three patients had lymphoid leukemia or myeloid leukemia, 24 patients had non-Hodgkin's lymphoma, 19 patients had Hodgkin's disease and 37 patients had other diseases, such as aplastic anemia, myelodysplasia or non-hematologic malignancy. The majority of patients (223 out of 243) had received prior chemotherapy with or without radiotherapy and/or immunotherapy prior to preparation for transplantation.
- One hundred day survival was improved in favor of the patients treated with Sargramostim after graft failure following either autologous or allogeneic BMT. In addition, the median survival was improved by greater than two-fold. The median survival of patients treated with Sargramostim after autologous failure was 474 days versus 161 days for the historical patients. Similarly, after allogeneic failure, the median survival was 97 days with Sargramostim treatment and 35 days for the historical controls. Improvement in survival was better in patients with fewer impaired organs.
- The MOF score is a simple clinical and laboratory assessment of seven major organ systems: cardiovascular, respiratory, gastrointestinal, hematologic, renal, hepatic and neurologic.10 Assessment of the MOF score is recommended as an additional method of determining the need to initiate treatment with Sargramostim in patients with graft failure or delay in engraftment following autologous or allogeneic BMT (see TABLE 5).

Factors that Contribute to Survival
- The probability of survival was relatively greater for patients with any one of the following characteristics: autologous BMT failure or delay in engraftment, exclusion of total body irradiation from the preparative regimen, a non-leukemic malignancy or MOF score ≤ two (zero, one or two dysfunctional organ systems). Leukemic subjects derived less benefit than other subjects.
How Supplied
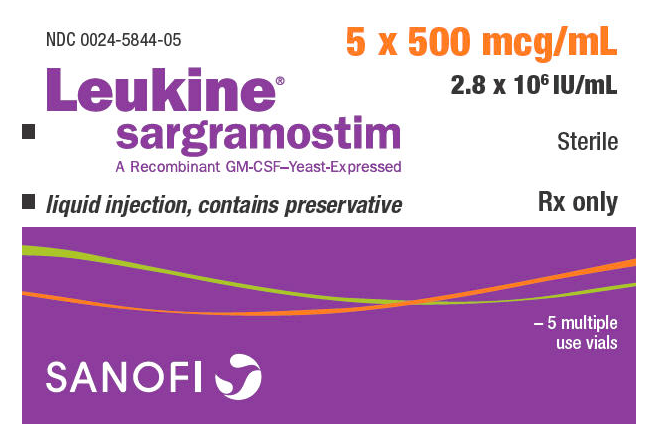
Liquid Sargramostim is available in vials containing 500 mcg/mL (2.8 × 106 IU/mL) sargramostim. Lyophilized Sargramostim is available in vials containing 250 mcg (1.4 × 106 IU/vial) sargramostim.
- Each dosage form is supplied as follows:
- Lyophilized Sargramostim: Carton of five vials of lyophilized Sargramostim 250 mcg (NDC 0024-5843-05)
- Liquid Sargramostim: Carton of one multiple-use vial; each vial contains 1 mL of preserved 500 mcg/mL liquid Sargramostim (NDC 0024-5844-01)
- Carton of five multiple-use vials; each vial contains 1 mL of preserved 500 mcg/mL liquid Sargramostim (NDC 0024-5844-05)
Storage
- Sargramostim should be refrigerated at 2–8°C (36–46°F). Do not freeze or shake. Do not use beyond the expiration date printed on the vial.
Images
Drug Images
{{#ask: Page Name::Sargramostim |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sargramostim |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Sargramostim Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Sargramostim interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Sargramostim Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.