Renal osteodystrophy
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Nazia Fuad M.D. , Parnian Jabbari
Synonyms and keywords:Chronic kidney disease- mineral bone disorder
Overview
Renal osteodystrophy (ROD) is within the broad spectrum of Chronic Kidney Disease (CKD)- Mineral Bone Disease (MBD). The disease occurs as a natural complication of the CKD and is characterized by abnormal levels and metabolism of calcium (Ca), phosphorus (Ph), parathyroid Hormone (PTH), and vitamin D, as well as calcification of soft tissues and bone turn over and mineralization abnormalities. Secondary hyperparathyroidism and 1,25-dihydroxycholecalciferol (vitamin D3) deficiency play a major role in ROD. Any factor leading to CKD is potentially a risk factor for ROD. Hypocalcemia, hyperphosphatemia, vitamin D deficiency, parathyroid gland hyperplasia and acidosis are the other contributors of ROD. Aluminum related ROD is mostly seen in patients who undergo dialysis. ROD is an important cause of morbidity, decreased quality of life, and extravascular calcifications that have been associated with increased cardiovascular mortality. Primary investigation of ROD includes measurement of blood levels of parathyroid hormone (PTH), calcium, phosphorus, alkaline phosphatase and bicarbonate. Imaging studies should focus on finding calcification in soft tissues. A bone biopsy is indicated if the results of biochemical markers are not consistent or when there is unexplained bone pain, or in case of presence of unexplained bone fractures. However, bone biopsies are infrequently used in clinical practice due to invasiveness and low cost-effectiveness. Common complications of ROD include bone fractures and vascular calcifications leading to atherosclerosis, coronary artery calcification, hypertension, left ventricular hypertrophy, and congestive heart failure (CHD). Extra-skeletal calcification can also affect the heart valves and the cardiac conduction system. Calcification of skin arterioles may lead to a condition of ischemia and necrosis of the skin known as calciphylaxis. Patients with renal osteodystrophy usually present with bone pain, arthralgia, chest pain, dyspnea, and palpitation. Physical examination of patients with renal osteodystrophy may include bone deformity, bone fracture, hypertension, ongestive heart failure, heart murmur, increased pulse pressure (due to aortic calcification) and skin ischemia and necrosis. In laboratory findings, serum calcium levels are typically low. Serum phosphorous is elevated depending on the stage of chronic kidney disease, dietary phosphorous, and use of phosphate binders. Alkaline phosphatase levels (total or bone-specific) are elevated and show increased osteoblastic activity. High levels of alkaline phosphatase are seen in severe osteitis fibrosa. Elecrocardiographic findings in patients with renal osteodystrophy include heart block and non-ST-elevation myocardial infarction. Radiographic findings are less sensitive for diagnosis compared to parathyroid hormone levels. Imaging is usually performed for patients with unexplained bone pain or fractures. Radiographic findings of osteitis fibrosa cystica include subperiosteal resorption. Resorptive loss of bone may be seen at the terminal phalanges, distal ends of the clavicles, and in the skull. Radiographs will show soft tissue calcification that involves the vasculature. Phosphate binders and supplemental calcium are mainly used for prevention and treatment of renal osteodystrophy. The major objective in the prevention and management of renal osteodystrophy is either prevention of hyperparathyroidism or its treatment if present.
Historical Perspective
- Renal osteodystrophy was first defined by Kidney Disease: Improving Global Outcomes (KDIGO) in 2006.
- It was discovered in the 1970s and 1980s, that aluminum in water that is used for dialysis and aluminum salts that are used as phosphate binders caused osteomalacia and an adynamic bone disease.
- The identification of these disorders led to define renal osteodystrophy.[1]
Classification
| Histologic Classification of Renal Osteodystrophy | |||
|---|---|---|---|
| Disorder | Description | Pathogenesis | Frequency (%) |
| Osteitis fibrosa | Peritrabecular fibrosis, increased
remodeling — resorption and formation. |
Secondary hyperparathyroidism, secondary
role of cytokines and growth factors |
50 |
| Osteomalacia | Increased osteoid, defective | Aluminum deposition, plus
unknown factors |
7 |
| Mixed disease | Features of both osteitis fibrosa
and osteomalacia |
Secondary hyperparathyroidism
and aluminum deposition, plus unknown factors |
13 |
| Mild disease | Slightly increased remodeling | Early or treated secondary | 3 |
| Adynamic renal
bone disease |
Hypocellular bone surfaces,
no remodeling |
Aluminum deposition, parathyroid hormone
suppression, and other factors (deficiency of bone growth factors or increased suppressors of bone remodeling) |
27 |
- After the bone pathology is assessed by histomorphometry, renal osteodystrophy can be subdivided according to TMV classification
- TMV uses three descriptions- bone turnover(T), bone mineralization(M) and bone volume(V).
- It helps to define the pathophysiology and to choose the right therapy.[2]
Pathophysiology
Overview of pathophysiology[3][4][2]:
In CKD, serum Ca levels decrease and serum Ph levels increase. Initially in the course of renal disease, compensatory mechanisms try to increase serum Ca and decrease serum Ph. These mechanisms include increased levels of fibroblast growth factor 23 (FGF23) which in turn increases urinary Ph excretion. On the other hand, increased PTH levels further increase urinary excretion of Ph. However, as the renal disease becomes chronic, these compensatory mechanisms do not respond any more and the characteristic features of ROD become evident [5]. Once an abnormality in serum levels of these minerals is established (decreased Ca and increased Ph), PTH levels increase and change bone metabolism via alterations in osteoblast and osteoclast activity. Early in CKD, due to increased FGF23, 1,25 (OH) vitamin D decreases which further leads to hyperparathyroidism (HPTH). However, some contributors to CKD-MBD alter before PTH levels are increased, an example of these contributors are sclerostin and FGF23 which are increased even before HPTH. HPTH can also insert its effects via the reduction of β-catenin which inhibits maturation of osteoblasts.
- PTH receptors are found on preosteoblasts, osteoblasts and osteocytes and increases their proliferation. (Osteoclasts do not have PTH receptors and are activated by preosteoblasts and osteoblasts.)
- Increased levels of PTH lead to increased bone resorption by osteoclasts [6] and osteitis fibrosa.
As a result, HPTH leads to high-turnover bone disease.
Many factors can contribute to low levels of PTH, such as increased dietary intake of Ca and Vit D, using Ph binders containing Ca dialysate. Low levels of PTH lead to low-turnover bone disease, also known as adynamic bone disease. Low PTH levels lead to excess circulating Ca (since Ca is not deposited in the bone). This excess Ca may lead to calcification of soft tissues.
Aluminum-based chelation of Ph during dialysis was among the common factors contributing to osteomalacia. However since replacement of aluminum with other chelators this factor is less prominent. [4][7][2]
CKD leads to uremia and hyperphosphatemia which change the pluripotent smooth muscle cells to osteoblasts. This coupled with increased Ca levels leads to calcification of soft tissues [8].
Overall, following factors contribute to vascular calcification [5]:
- Hypocalcemia and hyperphosphatemia
- Hyperparathyroidemia
- Matrix degradation and alteration of matrix proteins
- Apoptosis of smooth muscle cells
- Systemic inflammation
| Factors in the pathogenesis of hyperparathyroidism in chronic renal disease | |||||
|---|---|---|---|---|---|
| Phosphorus retention | Hypocalcemia | Low calcitriol | Skeletal
resistance |
Altered
parathyroid function | |
| ↓Renal mass | + | + | |||
| ↑Phosphorus | + | + | + | Unknown | |
| ↓Calcium | + | ||||
| ↓Calciterol | + | + | + | ||
| Skeletal resistance | + | ||||
| Desensitization to PTH | + | ||||
| ↓Vit D receptors | + | ||||
| Altered cell growth | + | ||||
| Acidosis | + | ||||
Causes
- The common causes of renal osteodystrophy are:[3][9]
- Chronic renal disease
- Hypocalcemia
- Hyperphosphatemia
- Increased FGF23 and sclerostin
- Vit D deficiency
- Parathyroid gland hyperplasia and hyperparathyroidism (HPTH is the most important factor in CKD-MBD)
- Acidosis
- Aluminum retention (in dialysis patients)
- Accumulation of β2M (beta-2 microglobulin) in bone and joints
Differentiating Renal Osteodystrophy from Other Diseases
- Renal osteodystrophy must be differentiated from the diseases that cause abnormal bone mineralization, unexplained bone fractures and bone pain:[3]
| Differential diagnosis of renal osteodystrophy | |||
|---|---|---|---|
| Calcium | Phosphate | Renal function | |
| Renal osteodystrophy | ↓ | ↑ | Markedly declined |
| Primary hyperparathyroidism | ↑ | Low to normal | Normal or slightly
reduced |
| Tertiary hyperparathyroidism | ↑ | Slightly elevated | Normal or slightly reduced |
| Osteoporosis | Normal | Normal | Normal |
| Vitamin D deficiency | ↓ | ↑ | Normal |
| Osteomalacia | ↓ | ↓ | Normal |
- Primary hyperparathyroidism will cause hypercalcemia, hyperparathyroidism, and normal-to-low phosphate in patients with either normal or slightly reduced renal function.
- Tertiary hyperparathyroidism causes hypercalcemia, hyperparathyroidism, and normal or slightly elevated phosphate in patients with long-term chronic kidney disease and mineral bone disorder (CKD-MBD).
- Osteoporosis, patients will have normal renal function.
- Vitamin D deficiency will cause normal or slight reduction in renal function.
Epidemiology and Demographics
- The prevelence of renal osteodystrophy is 8,000 per 100,000 in the adult population in US. Incidence of renal osteodystrophy increases in patients with chronic kidney disease who have glomerular filtration rate (GFR) less than 60 mL/min.[3]
- Prevalence in developing countries:
- The prevalence of renal osteodystrophy in developing countries is 24.4% to 63%.
- Aluminum, increased strontium levels and high levels of iron in the blood play a major role in the development of renal osteodystrophy in patients who undergo dialysis in developing countries.
- Extraskeletal manifestations of CKD-MBD (calcification of soft tissues) is observed in 1000 per 100,000 of CKD patients on dialysis.
Risk Factors
Any factor leading to CKD, indirectly leads to renal osteodystrophy. These factors include:
- Diabetes Mellitus
- Nephrotoxins such as alcohol and recreational drugs
- Acute kidney injury
- Hypertension
Some factors can enhance the effects of these risk factors such as Vitamin D deficiency and high-phosphate, low-calcium diet.
The major risk factors in the development of renal osteodystrophy are:[3]
- Chronic renal disease
- Dialysis (specifically with aluminum-based dialysate)
Natural History, Complications, and Prognosis
CKD leads to:
- hypocalcemia
- hyperphosphatemia (a predictor of cardiovascular and all-cause mortality)
- decreased Vit D levels
- hyperparathyroidism
- either increased bone turnover (due to HPTH and uremia) or adynamic bone disease
- calcification of soft tissues and vessels.
- HTN due to lessening ARB efficacy by increased levels of FGF23.
- cardiovascular disorders (uremic cardiomyopathy) (most important cause of mortality and morbidity in CKD).
- CKD can lead to ESRD and subsequent dialysis.
If left untreated, high-turnover bone disease leads to osteopenia and extraskeletal complications.
Common complications of renal osteodystrophy include:[3]
- Bone fractures
- Vascular calcification leading to atherosclerosis, coronary artery calcification, hypertension, left ventricular hypertrophy, and congestive heart failure.
- Extraskeletal calcification can also affect the heart valves and the cardiac conduction system.
- Calcification of skin arterioles may lead to a condition of ischemia and necrosis of the skin known as calciphylaxis.
Prognosis
- Renal osteodystrophy is associated with an increased risk of bone fractures, cardiovascular calcification, poor quality of life and increased morbidity and mortality in patients with chronic kidney disease.[3]
- However, prognosis is generally good after a renal transplant.
Diagnosis
Diagnostic Study of Choice
Bone biopsy
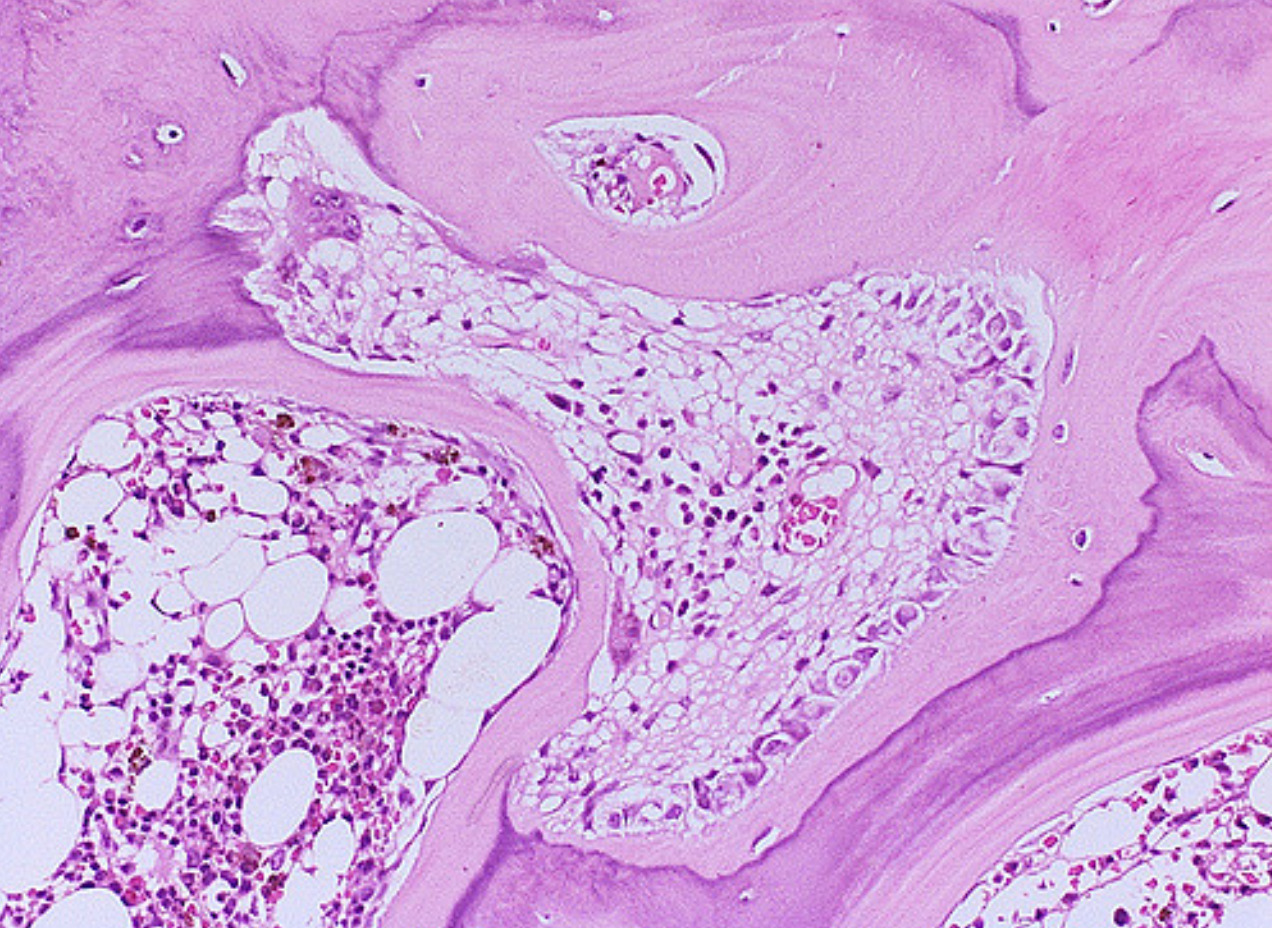
A definitive tool for diagnosis of renal osteodystrophy is bone biopsy according to KIDGO 2017 guidelines.[3]
- However, bone biopsies are infrequently performed because it is an invasive and expensive procedure.
- Bone biopsy results can be summerized in following pathologic categories.[1]
- In osteitis fibrosa there is increase amount of peritrabecular fibrosis, and increased amount of osteoclasts and osteoblasts.
- Osteomalacia demonstrate increased unmineralized bone matrix and decrease number of osteoclasts.
- Mixed uremic renal osteodystrophy shows areas of fibrosis and increased remodeling activity with poor mineralization and remodeling.
- Adynamic bone disease, shows absence of remodeling activity. The number of bone cells (osteoblasts and osteoclasts) is decreased.
Serum biomarkers:
The following biomarkers are used in the diagnosis of renal osteodystrophy:
- Serum calcium
- Serum phosphorous
- Alkaline phosphatase (total or bone-specific)
- Parathyroid hormone (PTH)
- Osteocalcin
- Tartrate-resistant acid phosphatase (TRAP)*
- Collagen degradation products (CDP)
- However, these products tend to accumulate in CKD regardless of MBD status*
- Pyrodinoline (PYD)*
- Dihydro-pyrodinoline (DPD)*
- PTH levels are considered to be the best noninvasive option to assess bone turnover.[4]
The following levels of PTH is used to describe the risk for different subtypes of renal osteodystrophy:[1]
- PTH <100 pg/mL means adynamic bone disease.
- Decreased risk of osteitis fibrosa cystica.
- PTH >500 pg/mL means osteitis fibrosa cystica and/or MUO (mixed uremic osteodystrophy).
- Intermediate PTH levels between 100 and 500 pg/mL Intermediate values may be due to normal or increased turnover or even reduced bone turnover.
* Still under investigation
History and Symptoms

- Patients with renal osteodystrophy are usually asymptomatic.
- When symptomatic, they usually present with:[2]
- Bone pain
- Arthralgia
- Chest pain
- Dyspnea
- Palpitation
Physical Examination
- Patients with renal osteodystrophy usually appear sick.
- Physical examination of patients with renal osteodystrophy may include:[3]
- Bone deformity
- Bone fracture
- Hypertension
- Congestive heart failure
- Heart murmur
- Increase pulse pressure (due to aortic calcification)
- Skin Ischemia and necrosis ( calciphylaxis)
Laboratory Findings
- Measurement of bone turnover on a bone biopsy is determined by labeling the bone with tetracycline. The procedure is done at two separate times approximately 2 weeks apart. The distance between the two areas of tetracycline deposition is measured and can be used to calculate bone growth.
- Serum calcium levels are typically low.
- Serum phosphorous is elevated depending on the stage of chronic kidney disease, dietary phosphorous, and use of phosphate binders.
- Alkaline phosphatase levels (total or bone-specific) are elevated and show increased osteoblastic activity. High levels are seen in severe osteitis fibrosa.
PTH(parathyroid hrmone) levels are the best noninvasive option for assessment of bone turnover.[2]
- The following parameters are used to define the risk for specific subtypes of renal osteodystrophy.[2]
- PTH <100 pg/mL is seen in adynamic bone disease and shows a decreased risk of osteitis fibrosa cystica and or MUO(mixed uremic osteodystrophy).
- PTH >450 pg/mL is present in osteitis fibrosa cystica and/or MUO (mixed uremic osteodystrophy).
- Intermediate PTH levels between 100 and 450 pg/mL. Intermediate values may be associated with normal or increased bone turnover or even reduced turnover.[3]
Electrocardiogram
- Electrocardiographic findings in patients with renal osteodystrophy may include:[3]
X-ray
- Routine radiographic screenings are not done for bone disease in patients with end-stage renal disease (ESRD).
- Radiographic findings are less sensitive for diagnosis than PTH levels.
- Imaging is usually performed for patients with unexplained bone pain or fractures.
Radiographic changes in ROD can be classified into four groups:
- Osteitis fibrosa
- Osteomalacia
- Osteosclerosis
- Soft-tissue calcification
- Radiographic findings of osteitis fibrosa cystica include:
- Subperiosteal resorption
- New bone formation especially at the radial aspect of the middle phalanges.
- Focal accumulation of giant cells (brown tumor) in facial bones, long bones, clavicle and phalanges.
- Varying density in skull bone ("salt and pepper" appearance)
- Radiographic findings of osteomalacia include:
- Psuedofractures in ischium and pubic rami
- Osteosclerosis is usually seen in spine, ribs and pelvis
- Extraeskeletal calcification is usually seen in cornea and conjuctiva and is directly associated with HPTH.
- Radiographs will show soft tissue calcification that involves the vasculature.[4]
- Resorptive loss of bone may be seen at the terminal phalanges, distal ends of the clavicles, and in the skull.
Echocardiography or Ultrasound
- Echocardiography will show:[4]
CT scan
- CT scan findings associated with renal osteodystrophy are the same that are related to chronic kidney disease.
MRI
- There are no specific MRI findings associated with renal osteodystrophy since associated changes are usually within the skeletal and bony tissue.
Other Diagnostic Studies
- DEXA bone densitometry will show low bone density.[2]
- Deferoxamine Challenge Test (DFO) can be used to rule out aluminum-related bone disease.
Treatment
Medical Therapy:
- Phosphate binders and supplemental calcium are mainly used for prevention and treatment of renal osteodystrophy.
Control of Serum Calcium
- Calcium malabsorption is seen in end-stage renal disease because of deficient 1,25-dihydroxycholecalciferol.[4]
- To prevent or suppress oversecretion of parathyroid hormone, calcium concentrations should be maintained at the high end of the normal range.
- In patients with calcium intakes of 800–1000 mg/day, additional calcium supplements or [][][,gcontaining medications should be avoided.
- Patients with total calcium intakes (>approx. 1000 mg/day) should be advised to decrease calcium intake.
- Patients with lower calcium intakes should be advised to increase calcium intake in foods,or take calcium supplements.
- Calcium-rich foods include dairy, dark green leafy vegetables, calcium-set tofu, and calcium-fortified orange juice.
- The timing of taking oral calcium is crucial as calcium taken between meals is more like a calcium supplement than a phosphate binder.
Control of Serum Phosphate
- A low-phosphate diet is crucial in the end-stage renal disease, to keep serum phosphate concentration within the normal limits.[3]
- Phosphate binder:
- Preferred regimen(1): Calcium carbonate, 500 mg PO q8h to be taken with each meal.
- Preferred regimen (2): Sevelamer carbonate 800 mg PO q8h with meal.
- Total dose of elemental calcium (including dietary sources and calcium-based phosphate binders) should not exceed 2000 mg daily in chronic kidney disease.
- Aluminum-containing phosphate binders should be avoided.
- Lanthanum carbonate is a non-calcium, non-aluminum phosphate binder proven to be effective when taken 1350 to 2250 mg/day.[11]
Use of Vit D analogue
- Calcitriol alfacalcidol, Doxercalciferol, and calcifediol
- Preferred regimen(1): Calcifediol , 30 mcg PO qHS for 3 months then increase the dose to 60mcg PO qHS is preferred regimen.
- Preferred regimen(2): Alfacalcidol, 0.25 mcg PO daily for 2 months.
- Preferred regimen(3): Doxercalciferol, 10 mcg PO 3 times/week at dialysis,may increase the dose by 2.5 mcg.
- Dialysis patients recieve 1 mcg intravenously during each dialysis session, 2-3 times weekly.
- Make sure serum calcium in <9.8 mg/dL before initiating vitamin D analogues.
- They decrease bone pain, improve bone histologic characteristics, and suppress parathyroid hormone secretion by increasing serum calcium concentrations and inhibiting parathyroid hormone gene transcription.[4][3]
Surgery
The treatment for renal osteodystrophy is medical therapy. Surgery is considered under the following circumstances:
- Subtotal parathyroidectomy (if PTH > 800 pg/ml).
- Renal transplant as final treatment approach.[3]
Primary Prevention
- Early diagnosis and treatment of hyperparathyroid patients.
- Reducing exacerbating factors such as smoking, uncontrolled diabetes, high-phosphate, low-calcium diet and sedentary life style.
- Early diagnosis and treatment of renal diseases to prevent chronic renal failure and consequently renal osteodystrophy.[4]
Secondary Prevention
- Vitamin D administration with every session of dialysis.
- Use of aluminum-free phosphate binders.[3]
References
- ↑ 1.0 1.1 1.2 1.3 Hruska, Keith A.; Epstein, Franklin H.; Teitelbaum, Steven L. (1995). "Renal Osteodystrophy". New England Journal of Medicine. 333 (3): 166–175. doi:10.1056/NEJM199507203330307. ISSN 0028-4793.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Moe, S.; Drüeke, T.; Cunningham, J.; Goodman, W.; Martin, K.; Olgaard, K.; Ott, S.; Sprague, S.; Lameire, N.; Eknoyan, G. (2006). "Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO)". Kidney International. 69 (11): 1945–1953. doi:10.1038/sj.ki.5000414. ISSN 0085-2538.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 https://www.orthopaedicsone.com/display/MSKMed/Renal+osteodystrophy
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Gonzalez, E. A.; Martin, K. J. (1995). "Renal osteodystrophy: pathogenesis and management". Nephrology Dialysis Transplantation. 10 (supp3): 13–21. doi:10.1093/ndt/10.supp3.13. ISSN 0931-0509.
- ↑ 5.0 5.1 Yi-Chou Hou, Chien-Lin Lu, Kuo-Cheng Lu. "Mineral bone disorders in chronic kidney disease".
- ↑ Lee SK, Lorenzo JA. "Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formation". https://academic.oup.com/endo/article/140/8/3552/2990646. External link in
|website=(help) - ↑ Moe, S.; Drüeke, T.; Cunningham, J.; Goodman, W.; Martin, K.; Olgaard, K.; Ott, S.; Sprague, S.; Lameire, N.; Eknoyan, G. (2006). "Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO)". Kidney International. 69 (11): 1945–1953. doi:10.1038/sj.ki.5000414. ISSN 0085-2538.
- ↑ Jorge B, Cannata-Andνa, Minerva Rodrνguez-Garcνa; et al. (2006). "ascular calcifications: Pathogenesis, management and impact on clinical outcomes". J Am Soc Nephrol. 17: 267–73.
- ↑ Nissenson, Allen (2009). Current diagnosis & treatment. New York: McGraw-Hill Medical. ISBN 978-0-07-144787-4.
- ↑ "renal psteodystrophy microscopic pathology". https://www.flickr.com/photos/bc_the_path/537039421/in/photolist-Pst7n. External link in
|website=(help) - ↑ Abdullah M.W. El-Kishawi*, A.M. El-Nahas (2006). "Renal Osteodystrophy: Review of the Disease and its Treatment". Saudi J Kidney Dis Transplant. 17 (3): 373–382.
Related Chapters
- osteoporosis
- osteopenia
- osteomalacia
- hyperparathyroidism
- multiple myeloma
- Soft tissue calcification including collagen vascular disease
- Hydroxyapatite crystal deposition disease
- Hypervitaminosis