Radium-223 chloride
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Radium-223 chloride is an antineoplastic agent that is FDA approved for the treatment of patients with castration-resistant prostate cancer, symptomatic bone metastases and no known visceral metastatic disease. Common adverse reactions include nausea, diarrhea, vomiting, and peripheral edema.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Recommended Dosage
- The dose regimen of Radium-223 chloride is 50 kBq (1.35 microcurie) per kg body weight, given at 4 week intervals for 6 injections. Safety and efficacy beyond 6 injections with Radium-223 chloride have not been studied. The volume to be administered to a given patient should be calculated using the:
- Patient’s body weight (kg)
- Dosage level 50 kBq/kg body weight or 1.35 microcurie/kg body weight
- Radioactivity concentration of the product (1,000 kBq/mL; 27 microcurie/mL) at the reference date
- Decay correction factor to correct for physical decay of radium-223.
- The total volume to be administered to a patient is calculated as follows:

- Immediately before and after administration, the net patient dose of administered Radium-223 chloride should be determined by measurement in an appropriate radioisotope dose calibrator that has been calibrated with a National Institute of Standards and Technology (NIST) traceable radium-223 standard (available upon request from Bayer) and corrected for decay using the date and time of calibration. The dose calibrator must be calibrated with nationally recognized standards, carried out at the time of commissioning, after any maintenance procedure that could affect the dosimetry and at intervals not to exceed one year.
Administration
- Administer Radium-223 chloride by slow intravenous injection over 1 minute.
- Flush the intravenous access line or cannula with isotonic saline before and after injection of Radium-223 chloride.
Instructions for Use / Handling
General warning
- Radium-223 chloride (an alpha particle-emitting pharmaceutical) should be received, used and administered only by authorized persons in designated clinical settings. The receipt, storage, use, transfer and disposal Radium-223 chloride are subject to the regulations and/or appropriate licenses of the competent official organization.
- Radium-223 chloride should be handled by the user in a manner which satisfies both radiation safety and pharmaceutical quality requirements. Appropriate aseptic precautions should be taken.
Radiation protection
- The administration of Radium-223 chloride is associated with potential risks to other persons (e.g., medical staff, caregivers and patient’s household members) from radiation or contamination from spills of bodily fluids such as urine, feces, or vomit. Therefore, radiation protection precautions must be taken in accordance with national and local regulations.
For drug handling
- Follow the normal working procedures for the handling of radiopharmaceuticals and use universal precautions for handling and administration such as gloves and barrier gowns when handling blood and bodily fluids to avoid contamination. In case of contact with skin or eyes, the affected area should be flushed immediately with water. In the event of spillage of Radium-223 chloride, the local radiation safety officer should be contacted immediately to initiate the necessary measurements and required procedures to decontaminate the area. A complexing agent such as 0.01 M ethylene-diamine-tetraacetic acid (EDTA) solution is recommended to remove contamination.
For patient care
- Whenever possible, patients should use a toilet and the toilet should be flushed several times after each use. When handling bodily fluids, simply wearing gloves and hand washing will protect caregivers. Clothing soiled with Radium-223 chloride or patient fecal matter or urine should be washed promptly and separately from other clothing.
- Radium-223 is primarily an alpha emitter, with a 95.3% fraction of energy emitted as alpha-particles. The fraction emitted as beta-particles is 3.6%, and the fraction emitted as gamma-radiation is 1.1%. The external radiation exposure associated with handling of patient doses is expected to be low, because the typical treatment activity will be below 8,000 kBq (216 microcurie). In keeping with the As Low As Reasonably Achievable (ALARA) principle for minimization of radiation exposure, it is recommended to minimize the time spent in radiation areas, to maximize the distance to radiation sources, and to use adequate shielding. Any unused product or materials used in connection with the preparation or administration are to be treated as radioactive waste and should be disposed of in accordance with local regulations.
- The gamma radiation associated with the decay of radium-223 and its daughters allows for the radioactivity measurement of Radium-223 chloride and the detection of contamination with standard instruments.
Instructions for preparation
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Radium-223 chloride is a ready-to-use solution and should not be diluted or mixed with any solutions. Each vial is for single use only.
Dosimetry
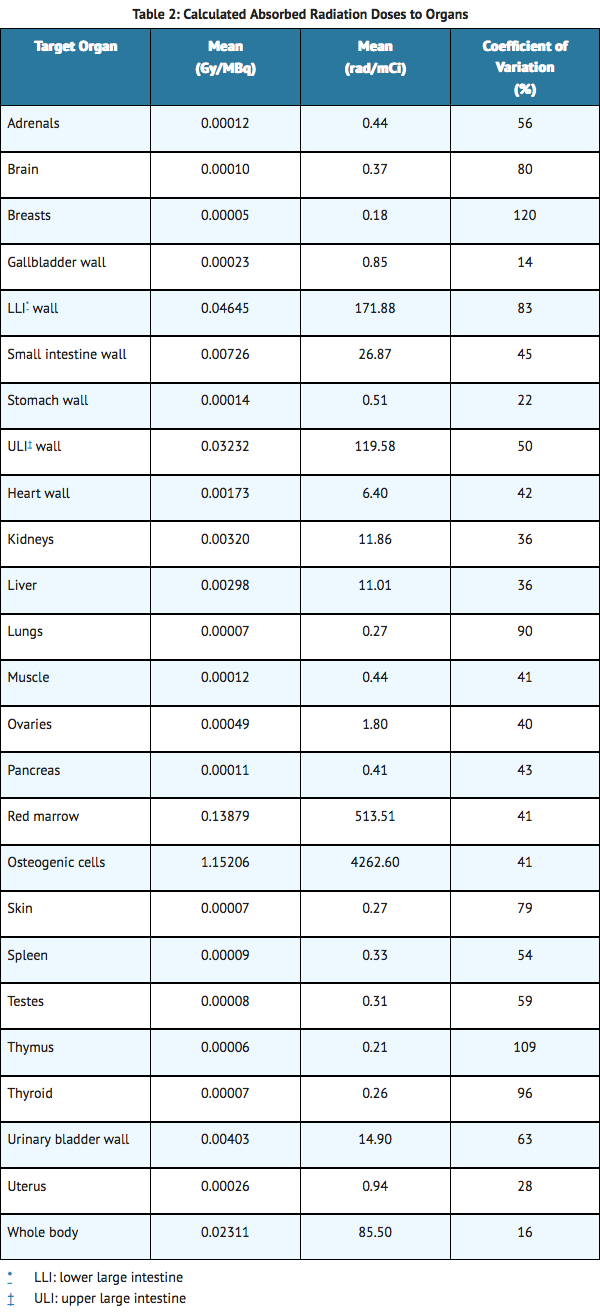
- The absorbed radiation doses in major organs were calculated based on clinical biodistribution data in five patients with castration-resistant prostate cancer. Calculations of absorbed radiation doses were performed using OLINDA/EXM (Organ Level INternal Dose Assessment/EXponential Modeling), a software program based on the Medical Internal Radiation Dose (MIRD) algorithm, which is widely used for established beta and gamma emitting radionuclides. For radium-223, which is primarily an alpha particle-emitter, assumptions were made for intestine, red marrow and bone/osteogenic cells to provide the best possible absorbed radiation dose calculations for Radium-223 chloride, considering its observed biodistribution and specific characteristics.
- The calculated absorbed radiation doses to different organs are listed in Table 2. The organs with highest absorbed radiation doses were bone (osteogenic cells), red marrow, upper large intestine wall, and lower large intestine wall. The calculated absorbed doses to other organs are lower.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Radium chloride in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Radium chloride in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Radium-223 chloride FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Radium chloride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Radium chloride in pediatric patients.
Contraindications
- Radium-223 chloride is contraindicated in pregnancy.
- Radium-223 chloride can cause fetal harm when administered to a pregnant woman based on its mechanism of action.
- Radium-223 chloride is not indicated for use in women.
- Radium-223 chloride is contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, apprise the patient of the potential hazard to the fetus
Warnings
Bone Marrow Suppression
- In the randomized trial, 2% of patients on the Radium-223 chloride arm experienced bone marrow failure or ongoing pancytopenia compared to no patients treated with placebo. There were two deaths due to bone marrow failure and for 7 of 13 patients treated with Radium-223 chloride, bone marrow failure was ongoing at the time of death. Among the 13 patients who experienced bone marrow failure, 54% required blood transfusions. Four percent (4%) of patients on the Radium-223 chloride arm and 2% on the placebo arm permanently discontinued therapy due to bone marrow suppression.
- In the randomized trial, deaths related to vascular hemorrhage in association with myelosuppression were observed in 1% of Radium-223 chloride-treated patients compared to 0.3% of patients treated with placebo. The incidence of infection-related deaths (2%), serious infections (10%), and febrile neutropenia (<1%) were similar for patients treated with Radium-223 chloride and placebo. Myelosuppression; notably thrombocytopenia, neutropenia, pancytopenia, and leukopenia; has been reported in patients treated with Radium-223 chloride. In the randomized trial, complete blood counts (CBCs) were obtained every 4 weeks prior to each dose and the nadir CBCs and times of recovery were not well characterized. In a separate single-dose phase 1 study of Radium-223 chloride, neutrophil and platelet count nadirs occurred 2 to 3 weeks after Radium-223 chloride administration at doses that were up to 1 to 5 times the recommended dose, and most patients recovered approximately 6 to 8 weeks after administration.
- Hematologic evaluation of patients must be performed at baseline and prior to every dose of Radium-223 chloride. Before the first administration of Radium-223 chloride, the absolute neutrophil count (ANC) should be ≥ 1.5 x 109/L, the platelet count ≥ 100 x 109/L and hemoglobin ≥ 10 g/dL. Before subsequent administrations of Radium-223 chloride, the ANC should be ≥ 1 x 109/L and the platelet count ≥ 50 x 109/L. If there is no recovery to these values within 6 to 8 weeks after the last administration of Radium-223 chloride, despite receiving supportive care, further treatment with Radium-223 chloride should be discontinued. Patients with evidence of compromised bone marrow reserve should be monitored closely and provided with supportive care measures when clinically indicated. Discontinue Radium-223 chloride in patients who experience life-threatening complications despite supportive care for bone marrow failure.
- The safety and efficacy of concomitant chemotherapy with Radium-223 chloride have not been established. Outside of a clinical trial, concomitant use with chemotherapy is not recommended due to the potential for additive myelosuppression. If chemotherapy, other systemic radioisotopes or hemibody external radiotherapy are administered during the treatment period, Radium-223 chloride should be discontinued.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In the randomized clinical trial in patients with metastatic castration-resistant prostate cancer with bone metastases, 600 patients received intravenous injections of 50 kBq/kg (1.35 microcurie/kg) of Radium-223 chloride and best standard of care and 301 patients received placebo and best standard of care once every 4 weeks for up to 6 injections. Prior to randomization, 58% and 57% of patients had received docetaxel in the Radium-223 chloride and placebo arms, respectively. The median duration of treatment was 20 weeks (6 cycles) for Radium-223 chloride and 18 weeks (5 cycles) for placebo.
- The most common adverse reactions (≥ 10%) in patients receiving Radium-223 chloride were nausea, diarrhea, vomiting, and peripheral edema (Table 3). Grade 3 and 4 adverse events were reported among 57% of Radium-223 chloride-treated patients and 63% of placebo-treated patients. The most common hematologic laboratory abnormalities in Radium-223 chloride-treated patients (≥ 10%) were anemia, lymphocytopenia, leukopenia, thrombocytopenia, and neutropenia.
- Treatment discontinuations due to adverse events occurred in 17% of patients who received Radium-223 chloride and 21% of patients who received placebo. The most common hematologic laboratory abnormalities leading to discontinuation for Radium-223 chloride were anemia (2%) and thrombocytopenia (2%).
Table 3 shows adverse reactions occurring in ≥ 2% of patients and for which the incidence for Radium-223 chloride exceeds the incidence for placebo.

Laboratory Abnormalities
Table 4 shows hematologic laboratory abnormalities occurring in > 10% of patients and for which the incidence for Radium-223 chloride exceeds the incidence for placebo.

- As an adverse reaction, grade 3-4 thrombocytopenia was reported in 6% of patients on Radium-223 chloride and in 2% of patients on placebo. Among patients who received Radium-223 chloride, the laboratory abnormality grade 3-4 thrombocytopenia occurred in 1% of docetaxel naïve patients and in 4% of patients who had received prior docetaxel. Grade 3-4 neutropenia occurred in 1% of docetaxel naïve patients and in 3% of patients who have received prior docetaxel.
Fluid Status
- Dehydration occurred in 3% of patients on Radium-223 chloride and 1% of patients on placebo. Radium-223 chloride increases adverse reactions such as diarrhea, nausea, and vomiting which may result in dehydration. Monitor patients’ oral intake and fluid status carefully and promptly treat patients who display signs or symptoms of dehydration or hypovolemia.
Injection Site Reactions
- Erythema, pain, and edema at the injection site were reported in 1% of patients on Radium-223 chloride.
Secondary Malignant Neoplasms
- Radium-223 chloride contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure may be associated with an increased risk of cancer and hereditary defects. Due to its mechanism of action and neoplastic changes, including osteosarcomas, in rats following administration of radium-223 dichloride, Radium-223 chloride may increase the risk of osteosarcoma or other secondary malignant neoplasms.
- However, the overall incidence of new malignancies in the randomized trial was lower on the Radium-223 chloride arm compared to placebo (<1% vs. 2%; respectively), but the expected latency period for the development of secondary malignancies exceeds the duration of follow up for patients on the trial.
Subsequent Treatment with Cytotoxic Chemotherapy
- In the randomized clinical trial, 16% patients in the Radium-223 chloride group and 18% patients in the placebo group received cytotoxic chemotherapy after completion of study treatments. Adequate safety monitoring and laboratory testing was not performed to assess how patients treated with Radium-223 chloride will tolerate subsequent cytotoxic chemotherapy.
Postmarketing Experience
There is limited information regarding Radium-223 chloride Postmarketing Experience in the drug label.
Drug Interactions
- No formal clinical drug interaction studies have been performed.
- Subgroup analyses indicated that the concurrent use of bisphosphonates or calcium channel blockers did not affect the safety and efficacy of Radium-223 chloride in the randomized clinical trial.
Use in Specific Populations
Pregnancy
- Radium-223 chloride can cause fetal harm when administered to a pregnant woman based on its mechanism of action. While there are no human or animal data on the use of Radium-223 chloride in pregnancy and Radium-223 chloride is not indicated for use in women, maternal use of a radioactive therapeutic agent could affect development of a fetus. Radium-223 chloride is contraindicated in women who are or may become pregnant while receiving the drug. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, apprise the patient of the potential hazard to the fetus and the potential risk for pregnancy loss. Advise females of reproductive potential to avoid becoming pregnant during treatment with Radium-223 chloride.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Radium-223 chloride in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Radium-223 chloride during labor and delivery.
Nursing Mothers
- Radium-223 chloride is not indicated for use in women. It is not known whether radium-223 dichloride is excreted in human milk. Because many drugs are excreted in human milk, and because of potential for serious adverse reactions in nursing infants from Radium-223 chloride, a decision should be made whether to discontinue nursing, or discontinue the drug taking into account the importance of the drug to the mother.
Pediatric Use
The safety and efficacy of Radium-223 chloride in pediatric patients have not been established.
- In single- and repeat-dose toxicity studies in rats, findings in the bones (depletion of osteocytes, osteoblasts, osteoclasts, fibro-osseous lesions, disruption/disorganization of the physis/growth line) and teeth (missing, irregular growth, fibro-osseous lesions in bone socket) correlated with a reduction of osteogenesis that occurred at clinically relevant doses beginning in the range of 20 – 80 kBq (0.541 - 2.16 microcurie) per kg body weight.
Geriatic Use
- Of the 600 patients treated with Radium-223 chloride in the randomized trial, 75% were 65 years of age and over and while 33% were 75 years of age and over. No dosage adjustment is considered necessary in elderly patients. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Radium-223 chloride with respect to specific gender populations.
Race
There is no FDA guidance on the use of Radium-223 chloride with respect to specific racial populations.
Renal Impairment
- No dedicated renal impairment trial for Radium-223 chloride has been conducted. Based on subgroup analyses in the randomized clinical trial, dose adjustment is not needed in patients with existing mild (creatinine clearance (CrCl) 60 to 89 mL/min) or moderate (CrCl 30 to 59 mL/min) renal impairment. No dose adjustment can be recommended for patients with severe renal impairment (CrCl less than 30 mL/min) due to limited data available (n = 2).
Hepatic Impairment
- No dedicated hepatic impairment trial for Radium-223 chloride has been conducted. Since radium-223 is neither metabolized by the liver nor eliminated via the bile, hepatic impairment is unlikely to affect the pharmacokinetics of radium-223 dichloride. Based on subgroup analyses in the randomized clinical trial, dose adjustment is not needed in patients with mild hepatic impairment. No dose adjustments can be recommended for patients with moderate or severe hepatic impairment due to lack of clinical data.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Radium-223 chloride in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Radium-223 chloride in patients who are immunocompromised.
Males of Reproductive Potential
- Contraception: Because of potential effects on spermatogenesis associated with radiation, advise men who are sexually active to use condoms and their female partners of reproductive potential to use a highly effective contraceptive method during and for 6 months after completing treatment with Radium-223 chloride.
- Infertility: There are no data on the effects of Radium-223 chloride on human fertility. There is a potential risk that radiation by Radium-223 chloride could impair human fertility
Administration and Monitoring
Administration
There is limited information regarding Radium-223 chloride Administration in the drug label.
Monitoring
There is limited information regarding Radium-223 chloride Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Radium-223 chloride and IV administrations.
Overdosage
There have been no reports of inadvertent overdosing of Radium-223 chloride during clinical studies.
- There is no specific antidote. In the event of an inadvertent overdose of Radium-223 chloride, utilize general supportive measures, including monitoring for potential hematological and gastrointestinal toxicity, and consider using medical countermeasures such as aluminum hydroxide, barium sulfate, calcium carbonate, calcium gluconate, calcium phosphate, or sodium alginate.
Single Radium-223 chloride doses up to 250 kBq (6.76 microcurie) per kg body weight were evaluated in a phase 1 clinical trial and no dose-limiting toxicities were observed.
Pharmacology

Mechanism of Action
- The active moiety of Radium-223 chloride is the alpha particle-emitting isotope radium-223 (as radium Ra 223 dichloride), which mimics calcium and forms complexes with the bone mineral hydroxyapatite at areas of increased bone turnover, such as bone metastases (see Table 2). The high linear energy transfer of alpha emitters (80 keV/micrometer) leads to a high frequency of double-strand DNA breaks in adjacent cells, resulting in an anti-tumor effect on bone metastases. The alpha particle range from radium-223 dichloride is less than 100 micrometers (less than 10 cell diameters) which limits damage to the surrounding normal tissue.
Structure
There is limited information regarding Radium-223 chloride Structure in the drug label.
Pharmacodynamics
- Compared with placebo, there was a significant difference in favor of Radium-223 chloride for all five serum biomarkers for bone turnover studied in a phase 2 randomized study (bone formation markers: bone alkaline phosphatase (ALP), total ALP and procollagen I N propeptide (PINP), bone resorption markers: C-terminal cross linking telopeptide of type I collagen (S-CTX-I) and type I collagen crosslinked C-telopeptide (ICTP)).
Pharmacokinetics
The pharmacokinetics of radium-223 dichloride in blood was linear in terms of dose proportionality and time independence in the dose range investigated (46 to 250 kBq [1.24 to 6.76 microcurie] per kg body weight).
Distribution
- After intravenous injection, radium-223 is rapidly cleared from the blood and is distributed primarily into bone or is excreted into intestine. Fifteen minutes post-injection, about 20% of the injected radioactivity remained in blood. At 4 hours, about 4% of the injected radioactivity remained in blood, decreasing to less than 1% at 24 hours after the injection. At 10 minutes post-injection, radioactivity was observed in bone and in intestine. At 4 hours post-injection, the percentage of the radioactive dose present in bone and intestine was approximately 61% and 49%, respectively. No significant uptake was seen in other organs such as heart, liver, kidneys, urinary bladder, and spleen at 4 hours post-injection.
Metabolism
- Radium-223 is an isotope that decays and is not metabolized.
Special Population
- Pediatric patients: Safety and effectiveness of Radium-223 chloride have not been established in children and adolescents below 18 years of age.
- Patients with hepatic impairment: No dedicated pharmacokinetic study in patients with hepatic impairment has been conducted. However, since radium-223 is not metabolized and there is no evidence of hepato-biliary excretion based on imaging data, hepatic impairment is not expected to affect the pharmacokinetics of radium-223 dichloride.
- Patients with renal impairment: No dedicated pharmacokinetic study in patients with renal impairment has been conducted. However, since excretion in urine is minimal and the major route of elimination is via the feces, renal impairment is not expected to affect the pharmacokinetics of radium-223 dichloride.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Animal studies have not been conducted to evaluate the carcinogenic potential of radium-223 dichloride. However, in repeat-dose toxicity studies in rats, osteosarcomas, a known effect of bone-seeking radionuclides, were observed at clinically relevant doses 7 to 12 months after the start of treatment. The presence of other neoplastic changes, including lymphoma and mammary gland carcinoma, was also reported in 12- to 15-month repeat-dose toxicity studies in rats.
- Genetic toxicology studies have not been conducted with radium-223 dichloride. However, the mechanism of action of radium-223 dichloride involves induction of double-strand DNA breaks, which is a known effect of radiation.
Animal studies have not been conducted to evaluate the effects of radium-223 dichloride on male or female fertility or reproductive function. Radium-223 chloride may impair fertility and reproductive function in humans based on its mechanism of action.
Clinical Studies
- The efficacy and safety of Radium-223 chloride were evaluated in a double-blind, randomized, placebo-controlled phase 3 clinical trial of patients with castration-resistant prostate cancer with symptomatic bone metastases. Patients with visceral metastases and malignant lymphadenopathy exceeding 3 cm were excluded. The primary efficacy endpoint was overall survival. A key secondary efficacy endpoint was time to first symptomatic skeletal event (SSE) defined as external beam radiation therapy (EBRT) to relieve skeletal symptoms, new symptomatic pathologic bone fracture, occurrence of spinal cord compression, or tumor-related orthopedic surgical intervention. There were no scheduled radiographic assessments performed on study. All patients were to continue androgen deprivation therapy. At the cut-off date of the pre-planned interim analysis, a total of 809 patients had been randomized 2:1 to receive Radium-223 chloride 50 kBq (1.35 microcurie)/kg intravenously every 4 weeks for 6 cycles (n = 541) plus best standard of care or matching placebo plus best standard of care (n = 268). Best standard of care included local EBRT, corticosteroids, antiandrogens, estrogens, estramustine or ketoconazole. Therapy was continued until unacceptable toxicity or initiation of cytotoxic chemotherapy, other systemic radioisotope, hemi-body EBRT or other investigational drug. Patients with Crohn’s disease, ulcerative colitis, prior hemibody radiation or untreated imminent spinal cord compression were excluded from the study. In patients with bone fractures, orthopedic stabilization was performed before starting or resuming treatment with Radium-223 chloride.
- The following patient demographics and baseline disease characteristics were balanced between the arms. The median age was 71 (range 44-94) with a racial distribution of 94% Caucasian, 4% Asian, 2% Black and <1% Other. Patients were enrolled predominantly from Europe (85%) with 4% of patients enrolled from North America. ECOG performance status was 0-1 in 86% of patients. Eighty-five percent of patients had 6 or more bone scan lesions and of those 40% had > 20 lesions or a superscan. Opiate pain medications were used for cancer-related pain in 54% of patients, non-opiate pain medications in 44% of patients and no pain medications in 2% of patients. Patients were stratified by baseline ALP, bisphosphonate use, and prior docetaxel exposure. Prior bisphosphonates were used by 41% of patients and 58% had received prior docetaxel. During the treatment period, 83% of Radium-223 chloride patients and 82% of placebo patients received gonadotropin-releasing hormone agonists and 21% of Radium-223 chloride patients and 34% of placebo patients received concomitant antiandrogens. Use of systemic steroids (41%) and bisphosphonates (40%) was balanced between the arms.
- The pre-specified interim analysis of overall survival revealed a statistically significant improvement in patients receiving Radium-223 chloride plus best standard of care compared with patients receiving placebo plus best standard of care. An exploratory updated overall survival analysis performed before patient crossover with an additional 214 events resulted in findings consistent with the interim analysis (Table 5).

The Kaplan-Meier curves for overall survival based on the updated survival results are shown in Figure 1.

How Supplied
- Radium-223 chloride (radium Ra 223 dichloride injection) is supplied in single-use vials containing 6 mL of solution at a concentration of 1,000 kBq/mL (27 microcurie/mL) with a total radioactivity of 6,000 kBq/vial (162 microcurie/vial) at the reference date (NDC 50419-208-01).
Storage
- Store at room temperature, below 40° C (104° F). Store Radium-223 chloride in the original container or equivalent radiation shielding.
Images
Drug Images
{{#ask: Page Name::Radium-223 chloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Radium-223 chloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Radium-223 chloride Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Radium chloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Radium-223 chloride Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.