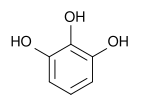
Pyrogallol
| |
| |
| Names | |
|---|---|
| Other names
1,2,3-Trihydroxybenzene
Pyrogallic acid, benzene-1,2,3-triol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| UNII | |
| |
| |
| Properties | |
| C6H6O3 | |
| Molar mass | 126.11 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
|
WikiDoc Resources for Pyrogallol |
|
Articles |
|---|
|
Most recent articles on Pyrogallol |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Pyrogallol at Clinical Trials.gov Clinical Trials on Pyrogallol at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Pyrogallol
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Pyrogallol Discussion groups on Pyrogallol Patient Handouts on Pyrogallol Directions to Hospitals Treating Pyrogallol Risk calculators and risk factors for Pyrogallol
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Pyrogallol |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Pyrogallol is an organic compound with the formula C6H3(OH)3. It is a white solid although because of its sensitivity toward oxygen, samples are typically brownish.[1] It is one of three isomeric benzenetriols.
Production, occurrence, reactions
It is produced in a manner it was first prepared by Scheele (1786): heating gallic acid. Presently gallic acid is obtained from tannin. Heating induces decarboxylation:
 Because tannin is expensive, many alternative routes have been devised
An alternate preparation involves treating para-chlorophenoldisulphonic acid with potassium hydroxide, a variant on the time-honored route to phenols from sulfonic acids.
Because tannin is expensive, many alternative routes have been devised
An alternate preparation involves treating para-chlorophenoldisulphonic acid with potassium hydroxide, a variant on the time-honored route to phenols from sulfonic acids.
The aquatic plant Myriophyllum spicatum produces pyrogallic acid.[2]
When in alkaline solution, it absorbs oxygen from the air, turning brown from a colourless solution. It can be used in this way to calculate the amount of oxygen in air, notably via the use of the Orsat apparatus.
Uses
One can find its uses in hair dying, dying of suturing materials and for oxygen absorption in gas analysis. It also has antiseptic properties. Pyrogallol was also used as a developing agent in black-and-white developers, but its use is largely historical except for special purpose applications. (Hydroquinone is more commonly used today.)
Safety
Pyrogallol use, e.g. in hair dye formulations, is declining because of concerns about its toxicity.[3] Its LD50 (oral, rat) is 300 mg/kg.[1]
See also
References
- ↑ 1.0 1.1 Template:Cite doi
- ↑ Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa. Satoshi Nakai, Yutaka Inoue, Masaaki Hosomi and Akihiko Murakami, Water Research, Volume 34, Issue 11, 1 August 2000, Pages 3026–3032, doi:10.1016/S0043-1354(00)00039-7
- ↑ Safety data for 1,2,3-trihydroxybenzene