Pyridostigmine Bromide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Pyridostigmine Bromide is a cholinesterase inhibitor that is FDA approved for the treatment of myasthenia gravis. Common adverse reactions include nausea, vomiting, diarrhea, abdominal cramps, increased peristalsis, increased salivation, increased bronchial secretions, miosis diaphoresis, muscle cramps and weakness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Pyridostigmine Bromide Tablets is useful in the treatment of myasthenia gravis.
Dosage
- Pyridostigmine Bromide Tablets contain 60 mg pyridostigmine bromide each.
- The size and frequency of the dosage must be adjusted to the needs of the individual patient.
- The average dose is ten 60 mg tablets daily, or spaced to provide maximum relief when maximum strength is needed. In severe cases as many as 25 tablets a day may be required, while in mild cases one to six tablets a day may suffice.
- Note: For information on a diagnostic test for myasthenia gravis, and for the evaluation and stabilization of therapy, please see product literature on Tensilon® (edrophonium chloride).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Pyridostigmine Bromide in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Pyridostigmine Bromide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- There is limited information regarding FDA-Labeled Use of Pyridostigmine Bromide in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Pyridostigmine Bromide in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Pyridostigmine Bromide in pediatric patients.
Contraindications
- Pyridostigmine Bromide Tablets are contraindicated in mechanical intestinal or urinary obstruction, and particular caution should be used in its administration to patients with bronchial asthma. Care should be observed in the use of atropine for counteracting side effects, as discussed below.
Warnings
- Although failure of patients to show clinical improvement may reflect underdosage, it can also be indicative of overdosage. As is true of all cholinergic drugs, overdosage of Pyridostigmine Bromide Tablets may result in cholinergic crisis, a state characterized by increasing muscle weakness which, through involvement of the muscles of respiration, may lead to death. Myasthenic crisis due to an increase in the severity of the disease is also accompanied by extreme muscle weakness, and thus may be difficult to distinguish from cholinergic crisis on a symptomatic basis. Such differentiation is extremely important, since increases in doses of Pyridostigmine Bromide Tablets or other drugs of this class in the presence of cholinergic crisis or of a refractory or "insensitive" state could have grave consequences. Osserman and Genkins1 indicate that the differential diagnosis of the two types of crisis may require the use of Tensilon® (edrophonium chloride) as well as clinical judgment. The treatment of the two conditions obviously differs radically. Whereas the presence of myasthenic crisis suggests the need for more intensive anticholinesterase therapy, the diagnosis of cholinergic crisis, according to Osserman and Genkins,1 calls for the prompt withdrawal of all drugs of this type. The immediate use of atropine in cholinergic crisis is also recommended.
- Atropine may also be used to abolish or obtund gastrointestinal side effects or other muscarinic reactions; but such use, by masking signs of overdosage, can lead to inadvertent induction of cholinergic crisis.
- For detailed information on the management of patients with myasthenia gravis, the physician is referred to one of the excellent reviews such as those by Osserman and Genkins,2 Grob3 or Schwab.4,5
Adverse Reactions
Clinical Trials Experience
- The side effects of Pyridostigmine Bromide Tablets are most commonly related to overdosage and generally are of two varieties, muscarinic and nicotinic. Among those in the former group are nausea, vomiting, diarrhea, abdominal cramps, increased peristalsis, increased salivation, increased bronchial secretions, miosis and diaphoresis. Nicotinic side effects are comprised chiefly of muscle cramps, fasciculation and weakness. Muscarinic side effects can usually be counteracted by atropine, but for reasons shown in the preceding section the expedient is not without danger. As with any compound containing the bromide radical, a skin rash may be seen in an occasional patient. Such reactions usually subside promptly upon discontinuance of the medication.
Postmarketing Experience
- There is limited information regarding Postmarketing Experience of Pyridostigmine Bromide in the drug label.
Drug Interactions
There is limited information regarding Pyridostigmine Bromide Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- The safety of Pyridostigmine Bromide Tablets during pregnancy or lactation in humans has not been established. Therefore, use of Pyridostigmine Bromide Tablets in women who may become pregnant requires weighing the drug's potential benefits against its possible hazards to mother and child.
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pyridostigmine Bromide in women who are pregnant.
Labor and Delivery
- There is no FDA guidance on use of Pyridostigmine Bromide during labor and delivery.
Nursing Mothers
- There is no FDA guidance on the use of Pyridostigmine Bromide with respect to nursing mothers.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- There is no FDA guidance on the use of Pyridostigmine Bromide with respect to geriatric patients.
Gender
- There is no FDA guidance on the use of Pyridostigmine Bromide with respect to specific gender populations.
Race
- There is no FDA guidance on the use of Pyridostigmine Bromide with respect to specific racial populations.
Renal Impairment
- There is no FDA guidance on the use of Pyridostigmine Bromide in patients with renal impairment.
Hepatic Impairment
- There is no FDA guidance on the use of Pyridostigmine Bromide in patients with hepatic impairment.
Females of Reproductive Potential and Males
- There is no FDA guidance on the use of Pyridostigmine Bromide in women of reproductive potentials and males.
Immunocompromised Patients
- There is no FDA guidance one the use of Pyridostigmine Bromide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- There is limited information regarding Monitoring of Pyridostigmine Bromide in the drug label.
IV Compatibility
- There is limited information regarding IV Compatibility of Pyridostigmine Bromide in the drug label.
Overdosage
- There is limited information regarding Chronic Overdose of Pyridostigmine Bromide in the drug label.
Pharmacology
| |
| |
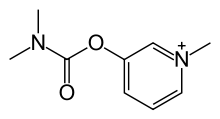
Pyridostigmine Bromide
| |
| Systematic (IUPAC) name | |
| 3-[(dimethylcarbamoyl)oxy]-1-methylpyridinium | |
| Identifiers | |
| CAS number | |
| ATC code | N07 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 181.212 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 7.6 +/- 2.4% |
| Metabolism | ? |
| Half life | 1.78 +/- 0.24hrs |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral, intravenous |
Mechanism of Action
- Pyridostigmine bromide inhibits the destruction of acetylcholine by cholinesterase and thereby permits freer transmission of nerve impulses across the neuromuscular junction. Pyridostigmine is an analog of neostigmine (Prostigmin®), but differs from it in certain clinically significant respects; for example, pyridostigmine is characterized by a longer duration of action and fewer gastrointestinal side effects.
Structure
- Pyridostigmine Bromide Tablets USP is an orally active cholinesterase inhibitor. Chemically, pyridostigmine bromide is 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate. Its structural formula is:

- Pyridostigmine bromide is available as tablets containing 60 mg pyridostigmine bromide; each tablet also contains lactose, silicon dioxide and stearic acid.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Pyridostigmine Bromide in the drug label.
Pharmacokinetics
- There is limited information regarding Pharmacokinetics of Pyridostigmine Bromide in the drug label.
Nonclinical Toxicology
- There is limited information regarding Nonclinical Toxicology of Pyridostigmine Bromide in the drug label.
Clinical Studies
- There is limited information regarding Clinical Studies of Pyridostigmine Bromide in the drug label.
How Supplied
- Tablets, 60 mg pyridostigmine bromide each, cross-scored on one side and engraved "OCEANSIDE 302" on the other, bottle of 100.
Storage
There is limited information regarding Pyridostigmine Bromide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Pyridostigmine Bromide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Pyridostigmine Bromide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Pyridostigmine Bromide in the drug label.
Precautions with Alcohol
- Alcohol-Pyridostigmine Bromide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- PYRIDOSTIGMINE BROMIDE®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "PYRIDOSTIGMINE BROMIDE- pyridostigmine bromide tablet".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Pyridostigmine Bromide
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Pyridostigmine Bromide |Label Name=Pyridostigmine Bromide11.png
}}
{{#subobject:
|Label Page=Pyridostigmine Bromide |Label Name=Pyridostigmine Bromide11.png
}}