Propranolol (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Propranolol (injection) is an beta-adrenergic blocker, Nonselective that is FDA approved for the treatment of cardiac arrhythmias. Common adverse reactions include diarrhea, vomiting, dizziness , sleep disorder, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Cardiac Arrhythmias
- Intravenous administration is usually reserved for life-threatening arrhythmias or those occurring under anesthesia.
Supraventricular arrhythmias
- Intravenous propranolol is indicated for the short-term treatment of supraventricular tachycardia, including Wolff-Parkinson-White syndrome and thyrotoxicosis, to decrease ventricular rate. Use in patients with atrial flutter or atrial fibrillation should be reserved for arrythmias unresponsive to standard therapy or when more prolonged control is required. Reversion to normal sinus rhythm has occasionally been observed, predominantly in patients with sinus or atrial tachycardia.
Ventricular tachycardias
- With the exception of those induced by catecholamines or digitalis, propranolol is not the drug of first choice. In critical situations when cardioversion techniques or other drugs are not indicated or are not effective, propranolol may be considered. If, after consideration of the risks involved, propranolol is used, it should be given intravenously in low dosage and very slowly, as the failing heart requires some sympathetic drive for maintenance of myocardial tone . Some patients may respond with complete reversion to normal sinus rhythm, but reduction in ventricular rate is more likely. Ventricular arrhythmias do not respond to propranolol as predictably as do the supraventricular arrhythmias. Intravenous propranolol is indicated for the treatment of persistent premature ventricular extrasystoles that impair the well-being of the patient and do not respond to conventional measures.
Tachyarrhythmias of digitalis intoxication
- Intravenous propranolol is indicated to control ventricular rate in life-threatening digitalis- induced arrhythmias. Severe bradycardia may occur .
Resistant tachyarrhythmias due to excessive catecholamine action during anesthesia
- Intravenous propranolol is indicated to abolish tachyarrhythmias due to excessive catecholamine action during anesthesia when other measures fail. These arrhythmias may arise because of release of endogenous catecholamines or administration of catecholamines. All general inhalation anesthetics produce some degree of myocardial depression. Therefore, when propranolol is used to treat arrhythmias during anesthesia, it should be used with extreme caution, usually with constant monitoring of the ECG and central venous pressure
Dosing Information
- The usual dose is 1 to 3 mg administered under careful monitoring, such as electrocardiography and central venous pressure. The rate of administration should not exceed 1 mg (1 mL) per minute to diminish the possibility of lowering blood pressure and causing cardiac standstill. Sufficient time should be allowed for the drug to reach the site of action even when a slow circulation is present. If necessary, a second dose may be given after two minutes. Thereafter, additional drug should not be given in less than four hours. Additional propranolol should not be given when the desired alteration in rate or rhythm is achieved. Transfer to oral therapy as soon as possible. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Propranolol (injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Propranolol (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Propranolol (injection) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Propranolol (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Propranolol (injection) in pediatric patients.
Contraindications
- Propranolol is contraindicated in 1) cardiogenic shock, 2) sinus bradycardia and greater than first degree block, 3) bronchial asthma, and 4) in patients with known hypersensitivity to propranolol hydrochloride.
Warnings
Cardiac Failure
- Sympathetic stimulation may be a vital component supporting circulatory function in patients with congestive heart failure, and its inhibition by beta blockade may precipitate more severe failure. Although beta-blockers should be avoided in overt congestive heart failure, some have been shown to be highly beneficial when used with close follow-up in patients with a history of failure who are well compensated and are receiving additional therapies, including diuretics as needed. Beta-adrenergic blocking agents do not abolish the inotropic action of digitalis on heart muscle.
Nonallergic Bronchospasm (e.g., Chronic Bronchitis, Emphysema)
- In general, patients with bronchospastic lung disease should not receive beta blockers. Propranolol should be administered with caution in this setting since it may block bronchodilation produced by endogenous and exogenous catecholamine stimulation of beta receptors.
Major Surgery
- The necessity or desirability of withdrawal of beta-blocking therapy prior to major surgery is controversial. It should be noted, however, that the impaired ability of the heart to respond to reflex adrenergic stimuli in propranolol-treated patients might augment the risks of general anesthesia and surgical procedures. Propranolol is a competitive inhibitor of beta-receptor agonists, and its effects can be reversed by administration of such agents, e.g., dobutamine or isoproterenol. However, such patients may be subject to protracted severe hypotension.
Diabetes and Hypoglycemia
- Beta-adrenergic blockade may prevent the appearance of certain premonitory signs and symptoms (pulse rate and pressure changes) of acute hypoglycemia, especially in labile insulin-dependent diabetics. In these patients, it may be more difficult to adjust the dosage of insulin. Propranolol therapy, particularly in infants and children, diabetic or not, has been associated with hypoglycemia especially during fasting, as in preparation for surgery. Hypoglycemia has been reported after prolonged physical exertion and in patients with renal insufficiency.
Thyrotoxicosis
- Beta-adrenergic blockade may mask certain clinical signs of hyperthyroidism. Therefore, abrupt withdrawal of propranolol may be followed by an exacerbation of symptoms of hyperthyroidism, including thyroid storm.
- Propranolol may change thyroid-function tests, increasing T4 and reverse T3 and decreasing T3.
Wolff-Parkinson-White Syndrome
- Beta-adrenergic blockade in patients with Wolff-Parkinson-White syndrome and tachycardia has been associated with severe bradycardia requiring treatment with a pacemaker. In one case this resulted after an initial 5 mg dose of intravenous propranolol.
Precautions
General
- Propranolol should be used with caution in patients with impaired hepatic or renal function. Propranolol is not indicated for the treatment of hypertensive emergencies. Beta-adrenergic receptor blockade can cause reduction of intraocular pressure. Patients should be told that propranolol might interfere with the glaucoma screening test. Withdrawal may lead to a return of elevated intraocular pressure.
Risk of Anaphylactic Reaction
- While taking beta blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
Angina Pectoris
- There have been reports of exacerbation of angina and, in some cases, myocardial infarction, following abrupt discontinuance of propranolol therapy. Therefore, when discontinuance of propranolol is planned, the dosage should be gradually reduced over at least a few weeks, and the patient should be cautioned against interruption or cessation of therapy without a physician's advice. If propranolol therapy is interrupted and exacerbation of angina occurs, it is usually advisable to reinstitute propranolol therapy and take other measures appropriate for the management of angina pectoris. Since coronary artery disease may be unrecognized, it may be prudent to follow the above advice in patients considered at risk of having occult atherosclerotic heart disease who are given propranolol for other indications.
Clinical Laboratory Tests
- In patients with hypertension, use of propranolol has been associated with elevated levels of serum potassium, serum transaminases and alkaline phosphatase. In severe heart failure, the use of propranolol has been associated with increases in Blood Urea Nitrogen.
Adverse Reactions
Clinical Trials Experience
- In a series of 225 patients, there were 6 deaths . Cardiovascular events (hypotension, congestive heart failure, bradycardia, and heart block) were the most common. *The only other event reported by more than one patient was nausea. Other adverse events for intravenous propranolol reported during post-marketing surveillance include cardiac arrest, dyspnea, and cutaneous ulcers. The following adverse events have been reported with use of formulations of sustained- or immediate-release oral propranolol and may be expected with intravenous propranolol.
- Cardiovascular: Bradycardia; congestive heart failure; intensification of AV block; hypotension; paresthesia of hands; thrombocytopenic purpura; arterial insufficiency, usually of the Raynaud type.
- Central Nervous System: Lightheadedness; mental depression manifested by insomnia, lassitude, weakness, fatigue; reversible mental depression progressing to catatonia; visual disturbances; hallucinations; vivid dreams; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance on neuropsychometrics. For immediate-release formulations, fatigue, lethargy, and vivid dreams appear doserelated.
- Gastrointestinal: Nausea, vomiting, epigastric distress, abdominal cramping, diarrhea, constipation, mesenteric arterial thrombosis, ischemic colitis.
- Allergic: Pharyngitis and agranulocytosis; erythematous rash, fever combined with aching and sore throat; laryngospasm and respiratory distress.
- Respiratory: Bronchospasm.
- Hematologic: Agranulocytosis, nonthrombocytopenic purpura, thrombocytopenic purpura.
- Autoimmune: In extremely rare instances, systemic lupus erythematosus has been reported.
- Miscellaneous: Alopecia, LE-like reactions, psoriasiform rashes, dry eyes, male impotence, and Peyronie's disease have been reported rarely. Oculomucocutaneous reactions involving the skin, serous membranes and conjunctivae reported for a beta-blocker (practolol) have not been associated with propranolol.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Propranolol (injection) in the drug label.
Drug Interactions
- Caution should be exercised when propranolol is administered with drugs that have an effect on CYP2D6, 1A2, or 2C19 metabolic pathways. Co-administration of such drugs with propranolol may lead to clinically relevant drug interactions and changes in its efficacy and/or toxicity .
Cardiovascular Drugs
- Antiarrhythmics
- Propafenone has negative inotropic and beta-blocking properties that can be additive to those of propranolol.
- Quinidine increases the concentration of propranolol and produces a greater degree of clinical beta-blockade and may cause postural hypotension.
- Disopyramide is a Type I antiarrhythmic drug with potent negative inotropic and chronotropic effects and has been associated with severe bradycardia, asystole and heart failure when administered with propranolol.
- Amiodarone is an antiarrhythmic agent with negative chronotropic properties that may be additive to those seen with propranolol.
- Caution should be exercised when administering propranolol with drugs that slow A-V nodal conduction, e.g., digitalis, lidocaine and calcium channel blockers.
- Calcium Channel Blockers
- Caution should be exercised when patients receiving a beta-blocker are administered a calcium-channel-blocking drug with negative inotropic and/or chronotropic effects. Both agents may depress myocardial contractility or atrioventricular conduction.
- There have been reports of significant bradycardia, heart failure, and cardiovascular collapse with concurrent use of verapamil and beta-blockers.
- Co-administration of propranolol and diltiazem in patients with cardiac disease has been associated with bradycardia, hypotension, high degree heart block, and heart failure.
- ACE Inhibitors
- When combined with beta-blockers, ACE inhibitors can cause hypotension, particularly in the setting of acute myocardial infarction.
- ACE inhibitors have been reported to increase bronchial hyperreactivity when administered with propranolol.
- The antihypertensive effects of clonidine may be antagonized by beta-blockers. Propranolol should be administered cautiously to patients withdrawing from clonidine.
- Alpha-blockers
- Prazosin has been associated with prolongation of first dose hypotension in the presence of beta-blockers.
- Postural hypotension has been reported in patients taking both beta-blockers and terazosin or doxazosin.
- Reserpine
- Patients receiving catecholamine-depleting drugs, such as reserpine, with propranolol should be closely observed for excess reduction of resting sympathetic nervous activity, which may result in hypotension, marked bradycardia, vertigo, syncopal attacks, or orthostatic hypotension.
- Administration of reserpine with propranolol may also potentiate depression.
- Inotropic Agents
- Patients on long-term therapy with propranolol may experience uncontrolled hypertension if administered epinephrine as a consequence of unopposed alpha-receptor stimulation.
- Epinephrine is therefore not indicated in the treatment of propranolol overdose
- Isoproterenol and Dobutamine
- Propranolol is a competitive inhibitor of beta-receptor agonists, and its effects can be reversed by administration of such agents, e.g., dobutamine or isoproterenol. Also, propranolol may reduce sensitivity to dobutamine stress echocardiography in patients undergoing evaluation for myocardial ischemia.
Non-Cardiovascular Drugs
- Non-Steroidal Anti-Inflammatory Drugs
- Nonsteroidal anti-inflammatory drugs (NSAIDS) have been reported to blunt the antihypertensive effect of beta-adrenoreceptor blocking agents.
- Administration of indomethacin with propranolol may reduce the efficacy of propranolol in reducing blood pressure and heart rate.
- Antidepressants
- The hypotensive effects of MAO inhibitors or tricyclic antidepressants may be exacerbated when administered with beta-blockers by interfering with the beta blocking activity of propranolol.
- Anesthetic Agents
- Methoxyflurane and trichloroethylene may depress myocardial contractility when administered with propranolol.
- Warfarin
- Administration of propranolol with warfarin increases the concentration of warfarin. Therefore, the prothrombin time should be monitored.
- Neuroleptic Drugs
- Hypotension and cardiac arrest have been reported with the concomitant use of propranolol and haloperidol.
- Thyroxine
- Thyroxine may result in a lower than expected T3 concentration when used concomitantly with propranolol.
Use in Specific Populations
Pregnancy
- Teratogenic Effects; In a series of reproductive and developmental toxicology studies, propranolol hydrochloride was given to rats by gavage or in the diet throughout pregnancy and lactation. At doses of 150 mg/kg/day, but not at doses of 80 mg/kg/day (equivalent to the MRHD on a body surface area basis), treatment was associated with embryotoxicity (reduced litter size and increased resorption rates) as well as neonatal toxicity (deaths). Propranolol hydrochloride also was administered (in the feed) to rabbits (throughout pregnancy and lactation) at doses as high as 150 mg/kg/day (about 5 times the maximum recommended human oral daily dose). No evidence of embryo or neonatal toxicity was noted.
- There are no adequate and well-controlled studies in pregnant women. Intrauterine growth retardation has been reported for neonates whose mothers received propranolol hydrochloride during pregnancy. Neonates whose mothers received propranolol hydrochloride at parturition have exhibited bradycardia, hypoglycemia and respiratory depression. Adequate facilities for monitoring these infants at birth should be available. Propranolol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Propranolol (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Propranolol (injection) during labor and delivery.
Nursing Mothers
- Propranolol is excreted in human milk. Caution should be exercised when propranolol is administered to a nursing woman
Pediatric Use
- Safety and effectiveness of propranolol in pediatric patients have not been established.
Geriatic Use
- Clinical studies of intravenous propranolol did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Elderly subjects have decreased clearance and a longer mean elimination halflife. These findings suggest that dose adjustment of propranolol injection may be required for elderly patients In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of the decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Propranolol (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Propranolol (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Propranolol (injection) in patients with renal impairment.
Hepatic Impairment
- Propranolol is extensively metabolized by the liver. Compared to normal subjects, patients with chronic liver disease have decreased clearance of propranolol, increased volume of distribution, decreased protein-binding and considerable variation in half-life. Consideration should be given to lowering the dose of intravenously administered propranolol in patients with hepatic insufficiency.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Propranolol (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Propranolol (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
There is limited information regarding Monitoring of Propranolol (injection) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Propranolol (injection) in the drug label.
Overdosage
- Propanolol is not significantly dialyzable. In the event of overdosage or exaggerated response, the following measures should be employed:
- Hypotension and bradycardia have been reported following propranolol overdose and should be treated appropriately. Glucagon can exert potent inotropic and chronotropic effects and may be particularly useful for the treatment of hypotension or depressed myocardial function after a propranolol overdose. Glucagon should be administered as 50 to 150 mcg/kg intravenously followed by continuous drip of 1 to 5 mg/hour for positive chronotropic effect. Isoproterenol, dopamine, or phosphodiesterase inhibitors may also be useful. Epinephrine, however, may provoke uncontrolled hypertension. Bradycardia can be treated with atropine or isoproterenol. Serious bradycardia may require temporary cardiac pacing. The electrocardiogram, pulse, blood pressure, neurobehavioral status and intake and output balance must be monitored. Isoproterenol and aminophylline may be useful for bronchospasm.
Pharmacology
| |
1 : 1 mixture (racemate)Propranolol
| |
| Systematic (IUPAC) name | |
| (RS)-1-(1-methylethylamino)-3-(1-naphthyloxy)propan-2-ol | |
| Identifiers | |
| CAS number | |
| ATC code | C07 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 259.34 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 26% |
| Metabolism | hepatic (extensive) |
| Half life | 4–5 hours |
| Excretion | renal <1% |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral, anal, IV |
Mechanism of Action
- Propranolol is a nonselective beta-adrenergic receptor blocking agent possessing no other autonomic nervous system activity. It specifically competes with beta-adrenergic receptor stimulating agents for available receptor sites. When access to beta-receptor sites is blocked by propranolol, chronotropic, inotropic, and vasodilator responses to beta-adrenergic stimulation are decreased proportionately. At doses greater than required for beta blockade, propranolol also exerts a quinidine-like or anesthetic-like membrane action, which affects the cardiac action potential. The significance of the membrane action in the treatment of arrhythmias is uncertain.
- The effects of propranolol are due to selective blockade of beta-adrenergic receptors, leaving alpha-adrenergic responses intact. There are two well-characterized subtypes of beta receptors (beta1 and beta2); propranolol interacts with both subtypes equally. Beta1- adrenergic receptors are found primarily in the heart. Blockade of cardiac beta1-adrenergic receptors leads to a decrease in the activity of both normal and ectopic pacemaker cells and a decrease in A-V nodal conduction velocity. All of these actions can contribute to antiarrhythmic activity and control of ventricular rate during arrhythmias. Blockade of cardiac beta1-adrenergic receptors also decreases the myocardial force of contraction and may provoke cardiac decompensation in patients with minimal cardiac reserve. Beta2-adrenergic receptors are found predominantly in smooth muscle—vascular, bronchial, gastrointestinal and genitourinary. *Blockade of these receptors results in constriction. Clinically, propranolol may exacerbate respiratory symptoms in patients with obstructive pulmonary diseases such as asthma and emphysema. Propranolol's beta blocking effects are attributable to its S(-) enantiomer.
Structure
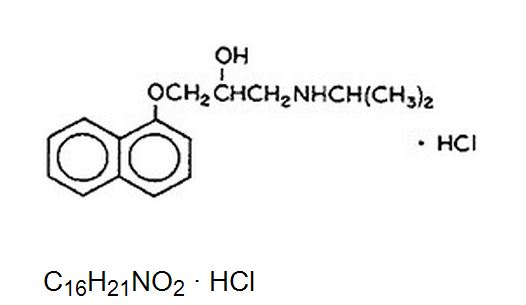
- Propranolol Hydrochloride Injection USP is a synthetic beta-adrenergic receptor blocking agent chemically described as (±)-1-(Isopropylamino)-3-(1-naphthyloxy)-2-propanol hydrochloride. Its structural formula is:
- Propranolol hydrochloride is a stable, white, crystalline solid which is readily soluble in water and ethanol. Its molecular weight is 295.80.
- Propranolol Hydrochloride Injection USP is available as a 1 mg/mL sterile injectable solution for intravenous administration. Each mL contains 1 mg of propranolol hydrochloride in Water for Injection. The pH is adjusted with citric acid. pH range is 2.8 to 4.0.
Pharmacodynamics
- As propranolol concentration increases, so does its beta-blocking effect, as evidenced by a reduction in exercise-induced tachycardia (n=6 normal volunteers).
Pharmacokinetics
- Distribution
- Propranolol has a distribution half-life (T1/2 alpha) of 5 to 10 minutes and a volume of distribution of about 4 to 5 L/kg. Approximately 90% of circulating propranolol is bound to plasma proteins. The binding is enantiomer-selective. The S-isomer is preferentially bound to alpha1 glycoprotein and the R-isomer is preferentially bound to albumin.
- Metabolism and Elimination
- The elimination half-life (T1/2 beta) is between 2 and 5.5 hours. Propranolol is extensively metabolized with most metabolites appearing in the urine. The major metabolites include propranolol glucuronide, naphthyloxylactic acid, and glucuronic acid and sulfate conjugates of 4-hydroxy propranolol. Following single-dose intravenous administration, side-chain oxidative products account for approximately 40% of the metabolites, direct conjugation products account for approximately 45 to 50% of metabolites, and ring oxidative products account for approximately 10 to 15% of metabolites. Of these, only the primary ring oxidative product (4-hydroxypropranolol) possesses beta-adrenergic receptor blocking activity.
- In vitro studies have indicated that the aromatic hydroxylation of propranolol is catalyzed mainly by polymorphic CYP2D6. Sidechain oxidation is mediated mainly by CYP1A2 and to some extent by CYP2D6. 4-hydroxy propranolol is a weak inhibitor of CYP2D6.Special Populations
Pediatric
- The pharmacokinetics of propranolol have not been investigated in patients under 18 years of age. Propranolol injection is not
recommended for treatment of cardiac arrhythmias in pediatric patients.
- Geriatric
- Elevated propranolol plasma concentrations, a longer mean elimination half-life (254 vs. 152 minutes), and decreased systemic clearance (8 vs. 13 mL/kg/min) have been observed in elderly subjects when compared to young subjects. However, the apparent volume of distribution seems to be similar in elderly and young subjects. These findings suggest that dose adjustment of propranolol injection may be required for elderly patients .
- Gender
- Intravenously administered propranolol was evaluated in 5 women and 6 men. When adjusted for weight, there were no gender-related differences in elimination half-life, volume of distribution, protein binding, or systemic clearance.
- Obesity
- In a study of intravenously administered propranolol, obese subjects had a higher AUC (161 versus 109 hr•mcg/L) and lower total clearance than did non-obese subjects. Propranolol plasma protein binding was similar in both groups.
- Renal Insufficiency
- The pharmacokinetics of propranolol and its metabolites were evaluated in 15 subjects with varying degrees of renal function after propranolol administration via the intravenous and oral routes. When compared with normal subjects, an increase in fecal excretion of propranolol conjugates was observed in patients with increased renal impairment. Propranolol was also evaluated in 5 patients with chronic renal failure, 6 patients on regular dialysis, and 5 healthy subjects, following a single oral dose of 40 mg of propranolol. The peak plasma concentrations (Cmax) of propranolol in the chronic renal failure group were 2- to 3-fold higher (161 ng/mL) than those observed in the dialysis patients (47 ng/mL) and in the healthy subjects (26 ng/mL). Propranolol plasma clearance was also reduced in the patients with chronic renal failure. Chronic renal failure has been associated with a decrease in drug metabolism via downregulation of hepatic cytochrome P450 activity.
- Hepatic Insufficiency
- Propranolol is extensively metabolized by the liver. In a study conducted in 6 normal subjects and 20 patients with chronic liver disease, including hepatic cirrhosis, 40 mg of R-propranolol was administered intravenously. Compared to normal subjects, patients with chronic liver disease had decreased clearance of propranolol, increased volume of distribution, decreased protein-binding, and considerable variation in half-life. Caution should be exercised when propranolol is used in this population. Consideration should be given to lowering the dose of intravenous propranolol in patients with hepatic insufficiency .
- Thyroid Dysfunction
- No pharmacokinetic changes were observed in hyperthyroid or hypothyroid patients when compared to their corresponding euthyroid state. Dosage adjustment does not seem necessary in either patient population based on pharmacokinetic findings.
- Drug Interactions
- Interactions with Substrates, Inhibitors or Inducers of Cytochrome P-450 Enzymes
- Because propranolol's metabolism involves multiple pathways in the cytochrome P-450 system (CYP2D6, 1A2, 2C19), administration of propranolol with drugs that are metabolized by, or affect the activity (induction or inhibition) of one or more of these pathways may lead to clinically relevant drug interactions (see PRECAUTIONS,Drug Interactions).
- Substrates or Inhibitors of CYP2D6
- Blood levels of propranolol may be increased by administration of propranolol with substrates or inhibitors of CYP2D6, such as amiodarone, cimetidine, delavirdine, fluoxetine, paroxetine, quinidine, and ritonavir. No interactions were observed with either ranitidine or lansoprazole.
- Substrates or Inhibitors of CYP1A2
- Blood levels of propranolol may be increased by administration of propranolol with substrates or inhibitors of CYP1A2, such as imipramine, cimetidine, ciprofloxacin, fluvoxamine, isoniazid, ritonavir, theophylline, zileuton, zolmitriptan, and rizatriptan.
- Substrates or Inhibitors of CYP2C19
- Blood levels of propranolol may be increased by administration of propranolol with substrates or inhibitors of CYP2C19, such as fluconazole, cimetidine, fluoxetine, fluvoxamine, teniposide, and tolbutamide. No interaction was observed with omeprazole.
- Inducers of Hepatic Drug Metabolism
- Blood levels of propranolol may be decreased by administration of propranolol with inducers such as rifampin and ethanol. Cigarette smoking also induces hepatic metabolism and has been shown to increase up to 100% the clearance of propranolol, resulting in decreased plasma concentrations.
Cardiovascular Drugs
- Antiarrhythmics
- The AUC of propafenone is increased by more than 200% with co-administration of propranolol.
- The metabolism of propranolol is reduced by co-administration of quinidine, leading to a 2- to 3-fold increased blood concentrations and greater beta-blockade.
- Calcium Channel Blockers
- The mean Cmax and AUC of propranolol are increased respectively, by 50% and 30% by coadministration of nisoldipine and by 80% and 47% by co-administration of nicardipine.
- The mean values of Cmax and AUC of nifedipine are increased by 64% and 79%, respectively, by co-administration of propranolol.
- Propranolol does not affect the pharmacokinetics of verapamil and norverapamil. Verapamil does not affect the pharmacokinetics of propranolol.
Non-Cardiovascular Drugs
- Migraine Drugs
- Administration of zolmitriptan or rizatriptan with propranolol resulted in increased concentrations of zolmitriptan (AUC increased by 56% and Cmax by 37%) or rizatriptan (the AUC and Cmax were increased by 67% and 75%, respectively).
- Theophylline
- Co-administration of theophylline with propranolol decreases theophylline clearance by 33% to 52%.
- Benzodiazepines
- The pharmacokinetics of oxazepam, triazolam, lorazepam, and alprazolam are not affected by co-administration of propranolol.
- Neuroleptic Drugs
- Co-administration of propranolol at doses greater than or equal to 160 mg/day resulted in increased thioridazine plasma concentrations ranging from 50% to 370% and increased thioridazine metabolites concentrations ranging from 33% to 210%.
- Co-administration of chlorpromazine with propranolol resulted in increased plasma levels of both drugs (70% increase in propranolol concentrations).
- Anti-Ulcer Drugs
- Co-administration of propranolol with cimetidine, a non-specific CYP450 inhibitor, increased propranolol concentrations by about 40%. Co-administration with aluminum hydroxide gel (1200 mg) resulted in a 50% decrease in propranolol concentrations.
- Co-administration of metoclopramide with propranolol did not have a significant effect on propranolol's pharmacokinetics.
- Lipid Lowering Drugs
- Co-administration of cholesteramine or colestipol with propranolol resulted in up to 50% decrease in propranolol concentrations.
- Co-administration of propranolol with lovastatin or pravastatin decreased 20% to 25% the AUC of both, but did not alter their pharmacodynamics. Propranolol did not have an effect on the pharmacokinetics of fluvastatin.
- Warfarin
- Concomitant administration of propranolol and warfarin has been shown to increase warfarin bioavailability and increase prothrombin time.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In dietary administration studies in which mice and rats were treated with propranolol hydrochloride for up to 18 months at doses of up to 150 mg/kg/day, there was no evidence of drug-related tumorigenesis. On a body surface area basis, this dose in the mouse and rat is, respectively, about equal to and about twice the maximum recommended human oral daily dose (MRHD) of 640 mg propranolol hydrochloride. In a study in which both male and female rats were exposed to propranolol hydrochloride in their diets at concentrations of up to 0.05%, (about 50 mg/kg body weight and less than the MRHD), from 60 days prior to mating and throughout pregnancy and lactation for two generations, there were no effects on fertility. Based on differing results from Ames Tests performed by different laboratories, there is equivocal evidence for a genotoxic effect of propranolol hydrochloride in bacteria ( S. typhimurium strain TA 1538).
Clinical Studies
- In a series of 225 patients with supraventricular (n=145), ventricular (n=69), or both (n=11) arrythmias resistant to digitalis, intravenous propranolol hydrochloride was administered in single doses, averaging 1 to 5 mg. Approximately one-quarter of the patients with supraventricular arrhythmias (generally those with sinus or atrial tachycardia) reverted to normal sinus rhythm. About one-half had symptoms ameliorated either by a decrease in ventricular rate or an attenuation of frequency or severity of paroxysmal attacks. Approximately one-half of patients with ventricular arrhythmias (generally those with frequent PVCs) reverted to normal sinus rhythm or responded with a reduction in ventricular rate. Similar findings were seen in a series of 25 Bantu patients with atrial fibrillation (n=16), sinus tachycardia (n=5), and multifocal ventricular extrasystoles (n=9). In another series, 7 of 8 patients with digitalis-related tachyarrhythmia had ventricular rate decreases after intravenous propranolol. Similarly, limited clinical experience has shown that intravenous propranolol will slow the ventricular rate in patients with Wolff-Parkinson-White syndrome or with tachycardia associated with thyrotoxicosis. Onset of activity is usually within five minutes.
How Supplied
- Propranolol Hydrochloride Injection USP, 1 mg/mL, is supplied as a 1 mL single dose vial in a carton of 10. NDC 55390-003-10.
- Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Protect from freezing and excessive heat. Discard unused portion. Protect from light. Retain in carton until time of use.
Storage
There is limited information regarding Propranolol (injection) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Propranolol (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Propranolol (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Propranolol (injection) in the drug label.
Precautions with Alcohol
- Alcohol-Propranolol (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Inderal
- Inderal LA
- InnoPran XL
- Propranolol HCl Intensol
- Inderal XL
- Hemangeol
Look-Alike Drug Names
There is limited information regarding Propranolol (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Propranolol (injection) |Label Name=Propranolol (injection)02.png
}}
{{#subobject:
|Label Page=Propranolol (injection) |Label Name=Propranolol (injection)03.png
}}
