Penicillin G procaine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Penicillin G procaine is an antibiotic that is FDA approved for the treatment of severe infections in both adults and pediatric patients due to penicillin-G-susceptible microorganisms namely streptococci, pneumococci, syphilis, fusospirochetosis. Common adverse reactions include allergic reactions, pseudomembranous colitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Penicillin G procaine is indicated in the treatment of moderately severe infections in both adults and pediatric patients due to penicillin-G-susceptible microorganisms that are susceptible to the low and persistent serum levels common to this particular dosage form in the indications listed below. Therapy should be guided by bacteriological studies (including susceptibility tests) and by clinical response.
- NOTE: When high, sustained serum levels are required, aqueous penicillin G, either IM or IV, should be used.
- The following infections will usually respond to adequate dosages of intramuscular penicillin G procaine: Moderately severe to severe infections of the upper respiratory tract, skin and soft-tissue infections, scarlet fever, and erysipelas due to susceptible streptococci (Group A-without bacteremia).
- NOTE: Streptococci in Groups A, C, G, H, L, and M are very sensitive to penicillin G. Other groups, including Group D (enterococcus), are resistant. Aqueous penicillin is recommended for streptococcal infections with bacteremia.
- Moderately severe infections of the respiratory tract due to susceptible pneumococci.
- NOTE: Severe pneumonia, empyema, bacteremia, pericarditis, meningitis, peritonitis, and arthritis of pneumococcal etiology are better treated with aqueous penicillin G during the acute stage.
- Moderately severe infections of the skin and soft tissues due to susceptible staphylococci(penicillin G-susceptible).
- NOTE: Reports indicate an increasing number of strains of staphylococci resistant to penicillin G, emphasizing the need for culture and sensitivity studies in treating suspected staphylococcal infections. Indicated surgical procedures should be performed.
- Fusospirochetosis (Vincent's gingivitis and pharyngitis). Moderately severe infections of the oropharynx due to susceptible fusiform bacilli and spirochetes.
- NOTE: Necessary dental care should be accomplished in infections involving the gum tissue.
- Syphilis (all stages) due to susceptible Treponema pallidum.
- NOTE: This drug should not be used in the treatment of beta-lactamase producing organisms which include most strains of Neisseria gonorrhea.
- Yaws, Bejel, Pinta due to susceptible organisms.
- Penicillin G procaine is an adjunct to antitoxin for prevention of the carrier stage of diphtheria due to susceptible C. diphtheriae.
- Anthrax due to Bacillus anthracis, including inhalational anthrax (post-exposure): to reduce the incidence or progression of the disease following exposure to aerosolized Bacillus anthracis.
- Rat-bite fever due to susceptible Streptobacillus moniliformis and Spirillum minus organisms.
- Erysipeloid due to susceptible Erysipelothrix rhusiopathiae.
- Subacute bacterial endocarditis, only in extremely sensitive infections, due to susceptible Group A streptococci.
- Do not inject into or near an artery or nerve. Injection into or near a nerve may result in permanent neurologic damage.
- Penicillin G procaine (aqueous) is for intramuscular injection only.
- Administer by DEEP INTRAMUSCULAR INJECTION in the upper, outer quadrant of the buttock. In neonates, infants and small children, the midlateral aspect of the thigh may be preferable. When doses are repeated, vary the injection site.
- Because of the high concentration of suspended material in this product, the needle may be blocked if the injection is not made at a slow, steady rate.
- Pneumonia (pneumococcal), moderately severe (uncomplicated): 600,000 to 1,000,000 units daily.
- Streptococcal infections (Group A), moderately severe to severe tonsillitis, erysipelas, scarlet fever, upper respiratory tract, skin and soft tissue: 600,000 to 1,000,000 units daily for 10-day minimum.
- Staphylococcal infections, moderately severe to severe: 600,000 to 1,000,000 units daily.
- In pneumonia, streptococcal (Group A) and staphylococcal infections in pediatric patients under 60 pounds: 300,000 units daily.
- Bacterial endocarditis (Group A streptococci) only in extremely sensitive infections: 600,000 to 1,000,000 units daily.
- Penicillin G procaine is not recommended for prophylaxis against bacterial endocarditis. For prophylaxis against bacterial endocarditis in patients with congenital heart disease or rheumatic or other acquired valvular heart disease when undergoing dental procedures or surgical procedures of the upper respiratory tract, use penicillin V. For patients unable to take oral medications, aqueous penicillin G is recommended.
- Syphilis
- Primary, secondary, and latent with a negative spinal fluid in adults and pediatric patients over 12 years of age: 600,000 units daily for 8 days-total 4,800,000 units.
- Late (tertiary, neurosyphilis, and latent syphilis with positive spinal-fluid examination or no spinal-fluid examination): 600,000 units daily for 10 to 15 days-total 6 to 9 million units.
- Congenital syphilis under 70-lb. body weight: 50,000 units/kg/day for 10 days.
- Yaws, Bejel, and Pinta: Treatment as for syphilis in corresponding stage of disease.
- Diphtheria-adjunctive therapy with antitoxin: 300,000 to 600,000 units daily.
- Diphtheria carrier state: 300,000 units daily for 10 days.
- Anthrax-cutaneous: 600,000 to 1,000,000 units/day.
- Anthrax-inhalational (post-exposure): 1,200,000 units every 12 hours in adults, 25,000 units per kilogram of body weight (maximum 1,200,000 unit) every 12 hours in children. The available safety data for penicillin G procaine at this dose would best support a duration of therapy of 2 weeks or less. Treatment for inhalational anthrax (post-exposure) must be continued for a total of 60 days. Physicians must consider the risks and benefits of continuing administration of penicillin G procaine for more than 2 weeks or switching to an effective alternative treatment.
- Vincent's infection (fusospirochetosis): 600,000 to 1,000,000 units/day.
- Erysipeloid: 600,000 to 1,000,000 units/day.
- Streptobacillus moniliformis and Spirillum minus (rat-bite fever): 600,000 to 1,000,000 units/day.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Penicillin G procaine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Penicillin G procaine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Penicillin G procaine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Penicillin G procaine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Penicillin G procaine in pediatric patients.
Contraindications
- A previous hypersensitivity reaction to any penicillin is a contraindication.
Warnings
- Penicillin G procaine should only be prescribed for the indications listed in this insert.
- NOTE: This drug is no longer indicated in the treatment of gonorrhea.
Anaphylaxis
- SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC) REACTIONS HAVE BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY AND/OR A HISTORY OF SENSITIVITY TO MULTIPLE ALLERGENS. THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE REACTIONS WHEN TREATED WITH CEPHALOSPORINS. BEFORE INITIATING THERAPY WITH ANY PENICILLIN, CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS, CEPHALOSPORINS, OR OTHER ALLERGENS. IF AN ALLERGIC REACTION OCCURS, THE DRUG SHOULD BE DISCONTINUED AND APPROPRIATE THERAPY INSTITUTED. SERIOUS ANAPHYLACTIC REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT WITH EPINEPHRINE. OXYGEN, INTRAVENOUS STEROIDS, AND AIRWAY MANAGEMENT, INCLUDING INTUBATION, SHOULD ALSO BE ADMINISTERED AS INDICATED.
Pseudomembranous Colitis
- Pseudomembranous colitis has been reported with nearly all antibacterial agents, including penicillin G, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
- Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridium. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of "antibiotic-associated colitis."
- After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management of fluids and electrolytes, protein supplementation and treatment with an antibacterial drug clinically effective against C. difficile colitis.
Procaine Reactions
- Immediate toxic reactions to procaine may occur in some individuals, particularly when a large single dose is administered (4.8 million units). These reactions may be manifested by mental disturbances, including anxiety, confusion, agitation, depression, weakness, seizures, hallucinations, combativeness, and expressed "fear of impending death." The reactions noted in carefully controlled studies occurred in approximately one in 500 patients who received large doses of penicillin G procaine. Reactions are transient, lasting from 15 to 30 minutes.
Method of Administration
- Do not inject into or near an artery or nerve.
- Injection into or near a nerve may result in permanent neurological damage.
- Inadvertent intravascular administration, including inadvertent direct intra-arterial injection or injection immediately adjacent to arteries, of Penicillin G Procaine Injectable Suspension and other penicillin preparations has resulted in severe neurovascular damage, including transverse myelitis with permanent paralysis, gangrene requiring amputation of digits and more proximal portions of extremities, and necrosis and sloughing at and surrounding the injection site. Such severe effects have been reported following injections into the buttock, thigh, and deltoid areas. Other serious complications of suspected intravascular administration which have been reported include immediate pallor, mottling, or cyanosis of the extremity, both distal and proximal to the injection site, followed by bleb formation; severe edema requiring anterior and/or posterior compartment fasciotomy in the lower extremity. The above-described severe effects and complications have most often occurred in infants and small children. Prompt consultation with an appropriate specialist is indicated if any evidence of compromise of the blood supply occurs at, proximal to, or distal to the site of injection.1-9.
- Quadriceps femoris fibrosis and atrophy have been reported following repeated intramuscular injections of penicillin preparations into the anterolateral thigh.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Penicillin G procaine in the drug label.
Postmarketing Experience
Allergic Reactions
- Penicillin is a substance of low toxicity but does possess a significant index of sensitization. The following hypersensitivity reactions associated with use of penicillin have been reported: Skin rashes, ranging from maculopapular eruptions to exfoliative dermatitis; urticaria; serum-sicknesslike reactions, including chills, fever, edema, arthralgia, and prostration. Severe and often fatal anaphylaxis has been reported. As with other treatments for syphilis, the Jarisch-Herxheimer reaction has been reported.
- Procaine toxicity manifestations and hypersensitivity reactions have been reported.
Gastrointestinal
- Pseudomembranous colitis has been reported with the use of penicillin G. Onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment.
Drug Interactions
There is limited information regarding Penicillin G procaine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Teratogenic effects—Pregnancy Category B: Reproduction studies performed in the mouse, rat, and rabbit have revealed no evidence of impaired fertility or harm to the fetus due to penicillin G. Human experience with the penicillins during pregnancy has not shown any positive evidence of adverse effects on the fetus. There are, however, no adequate and well-controlled studies in pregnant women showing conclusively that harmful effects of these drugs on the fetus can be excluded. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Penicillin G procaine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Penicillin G procaine during labor and delivery.
Nursing Mothers
- Penicillins are excreted in human milk. Caution should be exercised when penicillins are administered to a nursing woman.
Pediatric Use
- Because of incompletely developed renal function in newborns, penicillin elimination may be delayed. Guidelines for administration of this drug to pediatric patients are presented in
Geriatic Use
- Clinical studies of penicillin G procaine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Penicillin G procaine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Penicillin G procaine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Penicillin G procaine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Penicillin G procaine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Penicillin G procaine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Penicillin G procaine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral.
Monitoring
- Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
IV Compatibility
There is limited information regarding IV Compatibility of Penicillin G procaine in the drug label.
Overdosage
There is limited information regarding Penicillin G procaine overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Penicillin G procaine Pharmacology in the drug label.
Mechanism of Action
Microbiology
- Penicillin G exerts a bactericidal action against penicillin-susceptible microorganisms during the stage of active multiplication. It acts through the inhibition of biosynthesis of cell-wall mucopeptide. It is not active against the penicillinase-producing bacteria, which include many strains of staphylococci. While in vitro studies have demonstrated the susceptibility of most strains. Penicillin G exerts high in vitro activity against staphylococci (except penicillinase-producing strains), streptococci (Groups A, C, G, H, L, and M), and pneumococci. Other organisms susceptible to penicillin G are Corynebacterium diphtheriae, Bacillus anthracis, Clostridium species, Actinomyces bovis, Streptobacillus moniliformis, Listeria monocytogenes, and Leptospira species. Treponema pallidum is extremely susceptible to the bactericidal action of penicillin G.
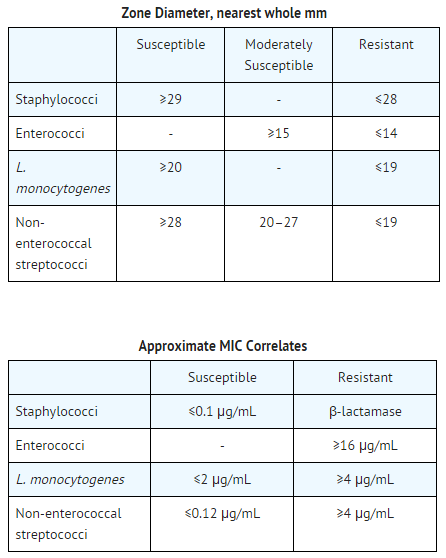
Susceptibility Testing
- Ten unit Penicillin G Susceptibility Discs may be used to determine microbial susceptibility to penicillin G using one of the following standard methods recommended by the National Committee for Laboratory Standards:
- M2-T4, "Performance Standards for Antimicrobial Disc Susceptibility Tests"
- M7-T2, "Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically"
Tests should be interpreted by the following criteria
- Interpretations of susceptible, intermediate, and resistant correlate zone size diameters with MIC values. A laboratory report of "susceptible" indicates that the suspected causative microorganism most likely will respond to therapy with penicillin G. A laboratory report of "resistant" indicates that the infecting microorganism most likely will not respond to therapy. A laboratory report of "moderately susceptible" indicates that the microorganism is most likely susceptible if a high dosage of penicillin G is used, or if the infection is such that high levels of penicillin G may be attained as in urine. A report of "intermediate" using the disc diffusion method may be considered an equivocal result, and dilution tests may be indicated.
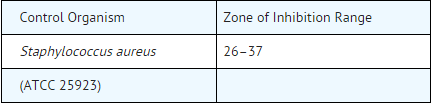
- Control organisms are recommended for susceptibility testing. Each time the test is performed the following organism should be included. The range for zones of inhibition is shown below:
Structure
- This product is designed to provide a stable aqueous suspension of penicillin G procaine, ready for immediate use. This eliminates the necessity for addition of any diluent, required for the usual dry formulation of injectable penicillin.
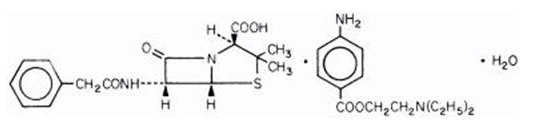
- Penicillin G procaine is chemically designated as (2S, 5R, 6R)-3,3-Dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid compound with 2-(diethylamino)ethyl p-aminobenzoate (1:1) monohydrate.
- Its molecular formula is C16H18N2O4S•C13H20N2O2•H2O with a molecular weight of 588.72. Its structural formula is as follows:
- Each syringe, 1,200,000 units (2 mL size) or 600,000 units (1 mL size), contains penicillin G procaine in a stabilized aqueous suspension with sodium citrate buffer; and as w/v, approximately 0.5% lecithin, 0.5% carboxymethylcellulose, 0.5% povidone, 0.1% methylparaben, and 0.01% propylparaben.
- Penicillin G Procaine Injectable Suspension is viscous and opaque.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Penicillin G procaine in the drug label.
Pharmacokinetics
- Penicillin G procaine is an equimolecular compound of procaine and penicillin G, administered intramuscularly as a suspension. It dissolves slowly at the site of injection, giving a plateau type of blood level at about 4 hours which falls slowly over a period of the next 15 to 20 hours.
- Approximately 60% of penicillin G is bound to serum protein. The drug is distributed throughout the body tissues in widely varying amounts. Highest levels are found in the kidneys with lesser amounts in the liver, skin, and intestines. Penicillin G penetrates into all other tissues to a lesser degree with a very small level found in the cerebrospinal fluid. With normal kidney function, the drug is excreted rapidly by tubular excretion. In neonates and young infants and in individuals with impaired kidney functions, excretion is considerably delayed. Approximately 60 to 90 percent of a dose of parenteral penicillin G is excreted in the urine within 24 to 36 hours.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Penicillin G procaine in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Penicillin G procaine in the drug label.
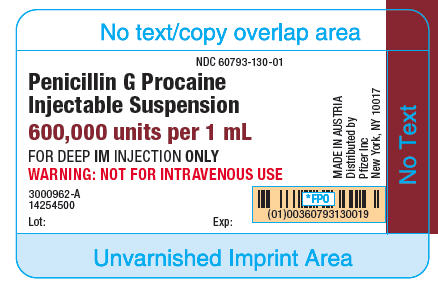
How Supplied
- Penicillin G Procaine Injectable Suspension is supplied in packages of 10 syringes as follows:
1 mL size, containing 600,000 units per syringe (21 gauge, thin-wall 1-1/2 inch needle), NDC 60793-130-10.
2 mL size, containing 1,200,000 units per syringe (21 gauge, thin-wall 1-1/2 inch needle), NDC 60793-131-10.
Storage
- Store in a refrigerator, 2° to 8°C (36° to 46°F).
Keep from freezing.
Images
Drug Images
{{#ask: Page Name::Penicillin G procaine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



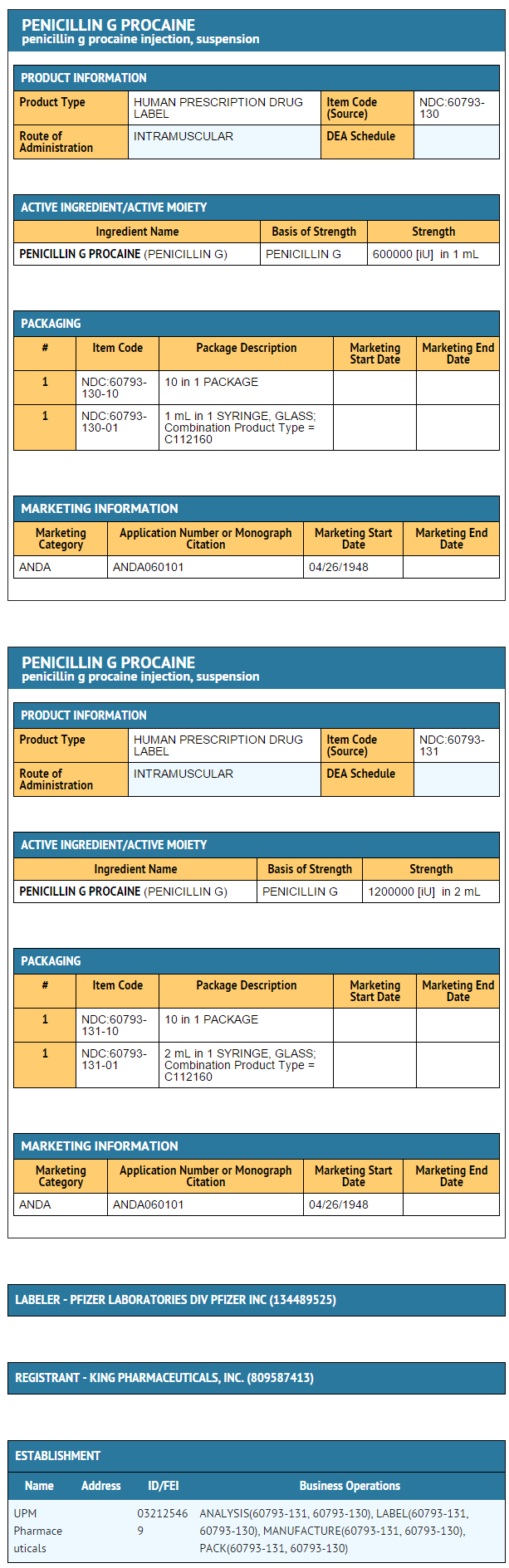
{{#ask: Label Page::Penicillin G procaine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Penicillin G procaine in the drug label.
Precautions with Alcohol
- Alcohol-Penicillin G procaine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- PENICILLIN G PROCAINE ®[1]
Look-Alike Drug Names
There is limited information regarding Penicillin G procaine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Penicillin G procaine |Label Name=Peni 01.jpg
}}
{{#subobject:
|Label Page=Penicillin G procaine |Label Name=Peni 02.jpg
}}
{{#subobject:
|Label Page=Penicillin G procaine |Label Name=Peni 03.jpg
}}
{{#subobject:
|Label Page=Penicillin G procaine |Label Name=Peni 04.jpg
}}
{{#subobject:
|Label Page=Penicillin G procaine |Label Name=DailyMed - PENICILLIN G PROCAINE- penicillin g procaine injection, suspension .png
}}