Prion
Template:DiseaseDisorder infobox
|
WikiDoc Resources for Prion |
|
Articles |
|---|
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Prion at Clinical Trials.gov Clinical Trials on Prion at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Prion
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Prion Risk calculators and risk factors for Prion
|
|
Healthcare Provider Resources |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
A prion (Template:IPAEng[1] — short for proteinaceous infectious particle (-on by analogy to virion) — is an infectious agent composed only of protein. They cause a number of diseases in a variety of animals, including BSE in cattle and CJD in humans. All known prion diseases affect the structure of the brain or other neural tissue, and all are currently untreatable and fatal. Mice genetically modified to avoid the symptoms are important models of study.[2] In general usage, prion can refer to both the theoretical unit of infection or the specific protein (e.g., PrP) that is thought to be the infective agent, whether or not it is in an infective state. Prion diseases can result from modification of a host-encoded glycoprotein (i.e. PrP)("protease resistant protein") which disrupts normal synaptic function.[3]
Prions are believed to infect and propagate by refolding abnormally into a structure which is able to convert normal molecules of the protein into the abnormally structured form. However, the term in itself does not preclude other mechanisms of transmission. All known prions induce the formation of an amyloid fold, in which the protein polymerizes into a fiber with a core consisting of tightly packed beta sheets. Other mechanisms may exist in yet undiscovered infectious protein particles. This altered structure renders them quite resistant to denaturation by chemical and physical agents, making disposal and containment of these particles difficult; even so, infectivity may be reduced by these treatments to a degree.
Proteins showing prion behaviour are also found in some fungi. Some fungal prions may not be associated with any disease; it is unknown whether these prions represent an evolutionary advantage for their hosts.
Discovery
Radiation biologist Tikvah Alper and physicist J.S. Griffith developed the theory in the 1960s that some transmissible spongiform encephalopathies are caused by an infectious agent made solely of protein.[4][5] This theory was developed to explain the discovery that the mysterious infectious agent causing the diseases scrapie and Creutzfeldt-Jakob Disease resisted ultraviolet radiation (which breaks down nucleic acids - present in viruses and all living things) yet responded to agents that disrupt proteins.
Francis Crick recognized the potential importance of the Griffith protein-only hypothesis for scrapie propagation in the second edition of his famous "Central dogma of molecular biology" (Nature. 1970 Aug 8;227(5258):561-3). While asserting that the flow of sequence information from protein to protein, or from protein to RNA and DNA was "precluded" by the dogma, he noted that Griffith's hypothesis was a potential difficulty (although it was not so promoted by Griffith). As the revised "dogma" was formulated, in part, to accommodate the then recent discovery of reverse transcription by Howard Temin and David Baltimore (Nobel Prize, 1975), proof of the protein-only hypothesis might have been seen as a sure bet for a future Prize.
Stanley B. Prusiner of the University of California, San Francisco announced in 1982 that his team had purified infectious material and that the infectious agent consisted mainly of a specific protein, although they had not managed to satisfactorily isolate the protein until two years after making his announcement.[6] Prusiner coined the word "prion" as a name for the infectious agent, by combining the first two syllables of the words proteinaceous and infectious.[7] While the infectious agent was named a prion, the specific protein that the prion was made of was named PrP, an abbreviation for "protease-resistant protein". Prusiner was awarded the Nobel Prize in Physiology or Medicine in 1997 for his research into prions.[8]
Structure
Isoforms
The protein that prions are made of is found throughout the body, even in healthy people and animals. However, the prion protein found in infectious material has a different structure and is resistant to proteases, the enzymes in the body that can normally break down proteins. The normal form of the protein is called PrPC, while the infectious form is called PrPSc — the C refers to 'cellular' or 'common' PrP, while the Sc refers to 'scrapie', a prion disease occurring in sheep. While PrPC is structurally well-defined, PrPSc is certainly polydisperse and defined at a relatively poor level. PrP can be induced to fold into other more-or-less well-defined isoforms in vitro, and their relationship to the form(s) that are pathogenic in vivo is not yet clear.
PrPC
PrPC is a normal protein found on the membranes of cells. Several topological forms of it exist; one cell surface form anchored via glycolipid and two transmembrane forms,[9] however its function has not been fully resolved.[10] PrPC is readily digested by proteinase K and can be liberated from the cell surface by the enzyme phosphatidyl inositol-specific phospholipase C, which cleaves the phosphatidyl inositol glycolipid anchor.[11] A typical yeast prion protein contains a core region (domain) with many repeats of the amino acids glutamine and asparagine. Normal yeast prion domains are flexible and lack a defined structure.[12]
PrPSc
The infectious isoform of PrPC, known as PrPSc, is able to catalyse the formation of other normal PrPC proteins into the infectious isoform by changing their conformation. Although the exact 3D structure of PrPSc is not known, there is increased β-sheet content in the diseased form of the molecule, replacing normal areas of α-helix.[13] Aggregations of these abnormal isoforms forms a highly structured amyloid fiber. The end of the fiber acts as a template for the free protein molecules, causing the fiber to grow. Small differences in the amino acid sequence of prion-forming regions lead to distinct structural features on the surface of prion fibers. As a result, only free protein molecules that are identical in amino acid sequence to the prion protein can be recruited into the growing fiber. The mammalian prion proteins do not resemble the prion proteins of yeast in their amino acid sequence, however, they are still known as PrPC and PrPSc and share basic structural features.
Function
PrP and long-term memory
There is evidence that PrP may have a normal function in maintenance of long term memory.[14] Maglio and colleagues have shown that mice without the genes for normal cellular PrP protein have altered hippocampal LTP.[15]
Prion disease
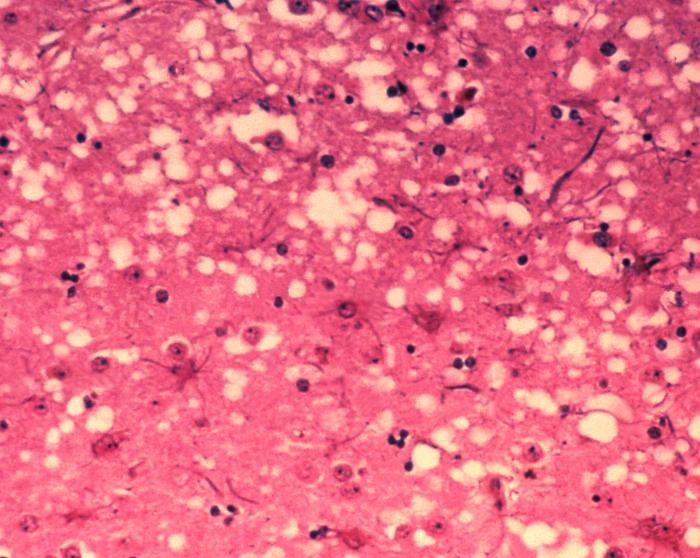
Prions cause neurodegenerative disease by aggregating extracellularly within the central nervous system to form plaques known as amyloids, which disrupt the normal tissue structure. This disruption is characterised by "holes" in the tissue with resultant spongy architecture due to the vacuole formation in the neurons.[16] Other histological changes include astrogliosis and the absence of an inflammatory reaction.[17] While the incubation period for prion diseases is generally quite long, once symptoms appear the disease progresses rapidly, leading to brain damage and death.[18] Neurodegenerative symptoms can include convulsions, dementia, ataxia (balance and coordination dysfunction), and behavioural or personality changes.
All known prion diseases, collectively called transmissible spongiform encephalopathies (TSEs), are untreatable and fatal.[19] However, a vaccine has been developed in mice that may provide insight into providing a vaccine in humans to resist prion infections.[20] Additionally, in 2006 scientists announced that they had genetically engineered cattle lacking a necessary gene for prion production - thus theoretically making them immune to BSE,[21] building on research indicating that mice lacking normally-occurring prion protein are resistant to infection by scrapie prion protein.[22]
Prions are able to affect a variety of different species, however the prions involved are somewhat species-specific: they are similar but not identical.[23] However overlap may occur; the human prion disease variant Creutzfeldt-Jakob disease is believed to be caused by a prion which typically infects cattle and is transmitted through infected meat.[24]
Metal ion interactions with prion proteins might be relevant to the progression of prion-mediated disease, based on epidemiological studies of clusters of prion disease in locales with low soil concentrations of copper.[25]
The following diseases are believed to be caused by prions.
- In animals:
- Scrapie in sheep and goats[26]
- Bovine spongiform encephalopathy (BSE) in cattle (known as mad cow disease)[26]
- Transmissible mink encephalopathy (TME) in mink[26]
- Chronic wasting disease (CWD) in elk and mule deer[26]
- Feline spongiform encephalopathy in cats[26]
- Exotic ungulate encephalopathy (EUE) in nyala, oryx and greater kudu[26]
- Spongiform encephalopathy of the ostrich[27]
- In humans:
- Creutzfeldt-Jakob disease (CJD)[26] and its varieties: iatrogenic Creutzfeldt-Jakob disease (iCJD), variant Creutzfeldt-Jakob disease (vCJD), familial Creutzfeldt-Jakob disease (fCJD), and sporadic Creutzfeldt-Jakob disease (sCJD)
- Gerstmann-Sträussler-Scheinker syndrome (GSS)[26]
- Fatal familial insomnia (fFI)[26]
- Sporadic fatal insomnia (sFI)[28]
- Kuru[26]
- Alpers syndrome[27]
Transmission
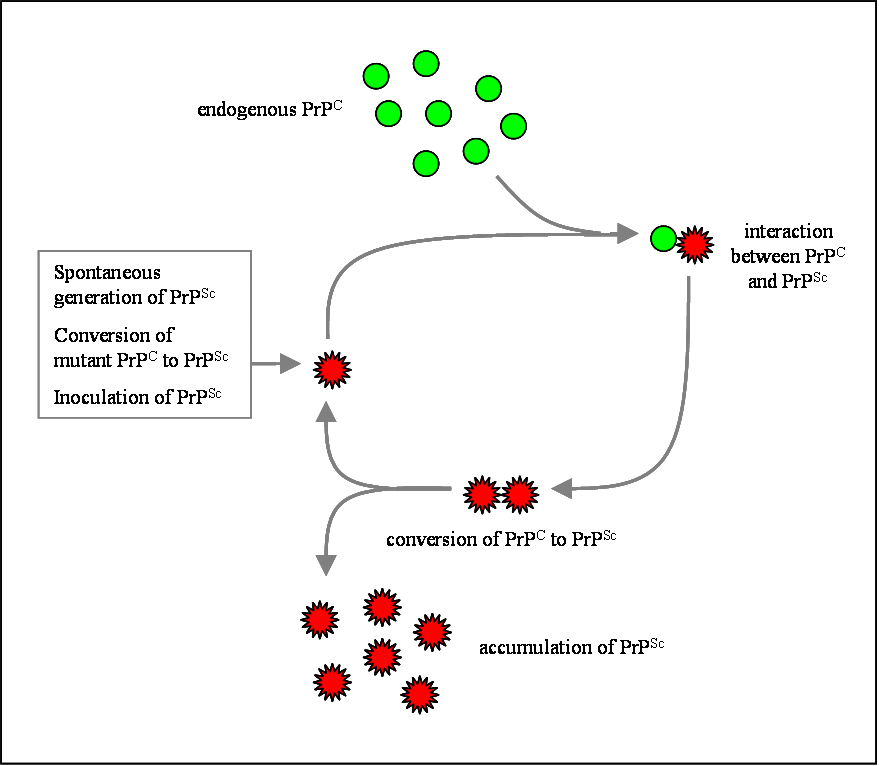
Although the identity and general properties of prions are now well-understood, the mechanism of prion infection and propagation remains mysterious. It is often assumed that the diseased form directly interacts with the normal form to make it rearrange its structure. One idea, the "Protein X" hypothesis, is that an as-yet unidentified cellular protein (Protein X) enables the conversion of PrPC to PrPSc by bringing a molecule of each of the two together into a complex.[29]
Current research suggests that the primary method of infection is through ingestion. Prions are deposited in the environment through the remains of dead animals and via urine, saliva, and other body fluids. They linger in the soil for at least three years by binding to clay and other minerals. This process in many cases makes the disease more transmissible.[30]
Sterilization
Infectious particles possessing nucleic acid are dependent upon it to direct their continued replication. Prions however, are infectious by their effect on normal versions of the protein. Therefore, sterilizing prions involves the denaturation of the protein to a state where the molecule is no longer able to induce the abnormal folding of normal proteins. However, prions are generally quite resistant to denaturation by proteases, heat, radiation, and formalin treatments,[31] although their infectivity can be reduced by such treatments.
Prions can be denatured by subjecting them to a temperatures of 134 degrees Celsius for 18 minutes in a pressurised steam autoclave.[32] Ozone sterilization is currently being studied as a potential method for prion deactivation.[33] Renaturation of a completely denatured prion to infectious status has not yet been achieved, however partially denatured prions can be renatured to an infective status under certain artificial conditions.[34]
Debate
Protein-only hypothesis
Prior to the discovery of prions, it was thought that all pathogens used nucleic acids to direct their replication. The "protein-only hypothesis" states that a protein structure can replicate without the use of nucleic acid. This was initially controversial as it contradicts the so-called "central dogma of modern biology," which describes nucleic acid as the central form of replicative information.
Evidence in favor of a protein-only hypothesis include:[35]
- No virus particles have been conclusively associated with prion diseases
- No nucleic acid has been conclusively associated with infectivity; agent is resistant to degradation by nucleases
- No immune response to infection
- PrPSc experimentally transmitted between one species and another results in PrPSc with the amino-acid sequence of the recipient species, suggesting that replication of the donor agent does not occur
- Level of infectivity is associated with levels of PrPSc
- PrPSc and PrPC do not differ in amino-acid sequence, therefore a PrPSc-specific nucleic acid is a redundant concept
- Familial prion disease occurs in families with a mutation in the PrP gene, and mice with PrP mutations develop prion disease despite controlled conditions where transmission is prevented
- PrPSc has been shown to arise from exposure of PrPC to molecules of PrPSc
Viral hypothesis
The protein-only hypothesis was initially met with skepticism and still has critics. For more than a decade, Yale University neuropathologist Laura Manuelidis has been proposing that prion diseases are caused instead by an unidentifiable "slow virus". In January 2007 she and her colleagues published an article in the Proceedings of the National Academy of Science reporting to have found the virus in 10%, or less, of their scrapie-infected cells in culture.[36][37]
Evidence in favor of a viral hypothesis include:[35]
- No bacteria or other living organisms have been found in prion-affected organisms, defaulting to the idea that a virus must be involved
- The long incubation and rapid onset of symptoms resembles some viral infections, such as HIV-induced AIDS
- Differences in prion infectivity, incubation, symptomology and progression among species resembles the "strain variation" seen between viruses, especially RNA viruses
- Familial prion disease is proposed to be due to genetic predisposition to the viral agent
Heavy Metal Poisoning hypothesis
Mark Purdey and Dr. David R. Brown have suggested that prions be accepted in the role of anti-oxidant molecules in conjunction with copper. Brown suggests that manganese can degrade the protein. This would be relevant to calling it a protein ion and declaring it an enzyme or ionophore.[38] Purdey cited epidemiological studies of clusters of prion disease in locales with low soil concentrations of copper as evidence.
Brown, an Oxford professor, agrees that banning cannibalism in cows was a justifiable course of action. It also follows from his work that carnivorous animals may be hazardous, because the pathogen may only show up as disease in animals with a long life.
Evidence in favor of a pollutant cause:
- Compounds of carbon and nitrogen vaporize at one thousand degrees Fahrenheit, but the agent for causing scrapie in sheep does not.
- Alzheimer's disease has similar symptoms, and has been attributed to excessive Aluminum.
- Copper deficiency and Manganese proficiency have been found in the environment of affected cattle.
Genetics
A gene for the normal protein has been isolated: the PRNP gene.[39] Some prion diseases can be inherited, and in all inherited cases there is a mutation in the PRNP gene. Many different PRNP mutations have been identified and it is thought that the mutations somehow make PrPC more likely to spontaneously change into the abnormal PrPSc form. Prion diseases are the only known diseases that can be sporadic, genetic, or infectious.
It should be noted that the same gene is responsible for spongiform encephalopathies which are not known to be transmissible, as well as some non-neurological diseases. Some require a mutation for transmission to occur, and there are respective mutations which can prevent or protect against transmission for most of the TSEs (eg. mutations leading to total absence of the PRNP gene or heterozygosity at codon 129 of the same gene).[16] The normal role of the prion gene has yet to be found, and so it is an area of considerable active research. Indeed gene knockout mice lacking the prion gene only exhibit subtle differences and seem to be incapable of acquiring spongiforme encephalopathy.
Prions in yeast and other fungi
Prion-like proteins that behave in a similar way to PrP are found naturally in some fungi and non-mammalian animals. A group at the Whitehead Institute has argued that some of the fungal prions are not associated with any disease state and may have a useful role; however, researchers at the NIH have also provided strong arguments demonstrating that fungal prions should be considered a diseased state. Research into fungal prions has given strong support to the protein-only hypothesis for mammalian prions, as it has been demonstrated that seeds extracted from cells with the prion state, can convert the normal form of the protein into the infectious form in vitro, and in the process, preserve the information corresponding to different strains of the prion state. It has also shed some light on prion domains, which are regions in a protein that promote the conversion. Fungal prions have helped to suggest mechanisms of conversion that may apply to all prions.
See also
References
- ↑ Template:OED
- ↑ Giovanna R. Mallucci (2007). "Targeting Cellular Prion Protein Reverses Early Cognitive Deficits and Neurophysiological Dysfunction in Prion-Infected Mice". Neuron. 53: 325-335.
- ↑ Prion protein is necessary for normal synaptic function Letters to Nature, Nature 370, 295 - 297 (28 July 1994); doi:10.1038/370295a0
- ↑ Alper T, Cramp W, Haig D, Clarke M (1967). "Does the agent of scrapie replicate without nucleic acid?". Nature. 214 (5090): 764–6. doi:10.1038/214764a0. PMID 4963878.
- ↑ Griffith J (1967). "Self-replication and scrapie". Nature. 215 (5105): 1043–4. doi:10.1038/2151043a0. PMID 4964084.
- ↑ Taubes, Gary (December 1986), "The game of name is fame. But is it science?", Discover, 7 (12): 28–41
- ↑ Prusiner, SB (1982). "Novel proteinaceous infectious particles cause scrapie". Science. 216 (4542): 136-144. doi:10.1126/science.278.5336.245. PMID 6801762.
- ↑ "The Nobel Prize in Physiology or Medicine 1997". NobelPrize.org. Retrieved 2007-05-11.
- ↑ DM Ou, CC Chen, CM Chen (2007). "Contact-Induced Structure Transformation in Transmembrane Prion Propagation". Biophysical Journal. 92 (8): 2704-2711.
- ↑ Shyng SL, Heuser JE, Harris DA (1994). "A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits". J Cell Biol. 125 (6): 1239-1250.
- ↑ Weissmann, C (2004). "The State of the Prion". Nature Reviews Microbiology. 2: 861-871.
- ↑ Stability, Folding, Dimerization, and Assembly Properties of the Yeast Prion Ure2p Biochemistry 2001, 40, 1764-1773
- ↑ "Conversion of alpha-helices into beta-sheets features in the formation of scrapie prion protein". PNAS USA. 90 (23): 10962–6. 1993 December 1. PMID 7902575. Check date values in:
|date=(help) - ↑ Shorter J, Lindquist S (2005). "Prions as adaptive conduits of memory and inheritance". Nat Rev Genet. 6 (6): 435–50. PMID 15931169.
- ↑ Maglio L, Perez M, Martins V, Brentani R, Ramirez O (2004). "Hippocampal synaptic plasticity in mice devoid of cellular prion protein". Brain Res Mol Brain Res. 131 (1–2): 58–64. PMID 15530652.
- ↑ 16.0 16.1 Cotran. Robbins Pathologic Basis of Disease. Philadelphia: W.B Saunders Company. 0-7216-7335-X. Unknown parameter
|coauthors=ignored (help) - ↑ Belay E. (1999). "Transmissible Spongiform Encephalopathies in Humans". Annu. Rev. Microbiol. 53: 283-314.
- ↑ "Prion Diseases". US Centers for Disease Control. Retrieved 2007-05-13.
- ↑ Gilch, Sabine; et al. (2001). "Intracellular re-routing of prion protein prevents propagation of PrPSc and delays onset of prion disease". The EMBO Journal (20): 3957-3966.
- ↑ New York University Medical Center and School of Medicine (2005-05-14). "Active Vaccine Prevents Mice From Developing Prion Disease". Science Daily. Retrieved 2007-05-08.
- ↑ Weiss, Rick (2007-01-01). "Scientists Announce Mad Cow Breakthrough". The Washington Post. Retrieved 2007-01-01.
- ↑ Büeler H, Aguzzi A, Sailer A, Greiner R, Autenried P, Aguet M, Weissmann C (1993). "Mice devoid of PrP are resistant to scrapie". Cell. 73 (7): 1339–47. PMID 8100741.
- ↑ Collinge J (2001). "Prion diseases of humans and animals: their causes and molecular basis". Annu Rev Neurosci. 24: 519–50. PMID 11283320.
- ↑ Ironside, JW (2006). "Variant Creutzfeldt-Jakob disease: risk of transmission by blood transfusion and blood therapies". Haemophilia. 12 (s1): 8-15.
- ↑ "2000-09-22, Normal Function of Prions, Statement to the BSE Inquiry" (PDF).
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 26.6 26.7 26.8 26.9 "90. Prions - ICTVdB Index of Viruses." (Website.) U.S. National Institutes of Health website. Retrieved on 2007-09-27.
- ↑ 27.0 27.1 Hussein, Mansour F. and Saud I. Al-Mufarrej. (2004.) "Prion Diseases: A Review; II. Prion Diseases in Man and Animals." Scientific Journal of King Faisal University (Basic and Applied Sciences), vol. 5, no. 2 1425, p. 139. Retrieved on 2007-09-27.
- ↑ {1999-05-28.) "BSE proteins may cause fatal insomnia." (News website.) BBC News. Retrieved on 2007-09-27.
- ↑ Telling G, Scott M, Mastrianni J, Gabizon R, Torchia M, Cohen F, DeArmond S, Prusiner S (1995). "Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein". Cell. 83 (1): 79–90. PMID 7553876.
- ↑ Johnson C, Pederson J, Chappell R, McKenzie D, Aiken J (2007). "Oral Transmissibility of Prion Disease is Enhanced by Binding to Soil Particles". PLoS Pathogens. 3 (7).
- ↑ Qin K, O'Donnell M, Zhao R (2006). "Doppel: more rival than double to prion". Neuroscience. 141 (1): 1–8. doi:10.1016/j.neuroscience.2006.04.057. PMID 16781817. Unknown parameter
|link=ignored (help) - ↑ Collins SJ, Lawson VA, Masters CL (2004). "Transmissible spongiform encephalopathies". Lancet. 363 (9402): 51–61. PMID 14723996.
- ↑ Ozone Sterilization - UK Health Protection Agency
- ↑ Weissmann C, Enari M, Klöhn PC, Rossi D, Flechsig E (2002). "Transmission of prions". Proc. Natl. Acad. Sci. U.S.A. 99 Suppl 4: 16378–83. PMID 12181490.
- ↑ 35.0 35.1 Baker & Ridley (1996). Prion Disease. New Jersey: Humana Press. 0-89603-342-2.
- ↑ "Pathogenic Virus Found in Mad Cow Cells". Yale. February 2 2007. Retrieved 2007-02-02. Check date values in:
|date=(help) - ↑ Laura Manuelidis, Zhoa-Xue Yu, Nuria Barquero, and Brian Mullins (February 6, 2007). "Cells infected with scrapie and Creutzfeldt–Jakob disease agents produce intracellular 25-nm virus-like particles". Proceedings of the National Academy of Sciences of the United States of America. 104 (6): 1965–1970. doi:10.1073/pnas.0610999104.
- ↑ 2000-09-22, Normal Function of Prions, Statement to the BSE Inquiry
- ↑ Oesch B, Westaway D, Wälchli M, McKinley M, Kent S, Aebersold R, Barry R, Tempst P, Teplow D, Hood L (1985). "A cellular gene encodes scrapie PrP 27-30 protein". Cell. 40 (4): 735–46. PMID 2859120.
External links
- Biography of Dr Prusiner
- Mammalian prions - 90.001.0.01 ICTVdb
- MicrobiologyBytes: Prion Diseases
- Prion Diseases and the BSE Crisis (1997). Article from Science magazine by Stanley Prusiner.
- Overview of prion biology from the Science Creative Quarterly
- Science Daily article on transmission of prions through soil
- The Pathological Protein - Mad Cow, Chronic Wasting, and Other Deadly Prion Diseases] (2003, updated online 2005). Philip Yam, Scientific American writer and news editor.
ar:بريون bg:Прион ca:Prió cs:Prion de:Prion et:Prioonid eo:Priono fa:پریون gl:Prión ko:프리온 hr:Prioni id:Prion ia:Prion it:Prione he:פריון lt:Prionas hu:Prion nl:Prion no:Prioner simple:Prion sk:Prión sr:Приони fi:Prioni sv:Prion th:พรีออน