Pivagabine
| |
| Names | |
|---|---|
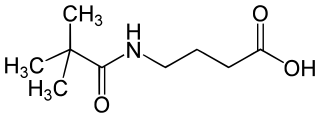
| IUPAC name
4-(2,2-Dimethylpropanoylamino)butanoic acid[citation needed]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| KEGG | |
| MeSH | N-trimethylacetyl-4-aminobutyric+acid |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H17NO3 | |
| Molar mass | 187.24 g·mol−1 |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
|
WikiDoc Resources for Pivagabine |
|
Articles |
|---|
|
Most recent articles on Pivagabine |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Pivagabine at Clinical Trials.gov Clinical Trials on Pivagabine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Pivagabine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Pivagabine Discussion groups on Pivagabine Patient Handouts on Pivagabine Directions to Hospitals Treating Pivagabine Risk calculators and risk factors for Pivagabine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Pivagabine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Pivagabine (Tonerg) is an antidepressant and anxiolytic drug which was introduced in Italy in 1997 for the treatment of depressive and maladaptive syndromes. But it was discontinued in Italy (according to Martindale). Originally believed to function as a prodrug to GABA,[1] pivagabine is now believed to act via its inhibitory effects on corticotropin-releasing factor (CRF).[2][3][4][5]
References
- ↑ Bianchi M, Quadro G, Mourier G, Galzigna L (1983). "Pharmacokinetics and in vitro effects of a 4-aminobutyric acid derivative with anticonvulsant action". Pharmacology. 27 (4): 237–40. doi:10.1159/000137876. PMID 6634934.
- ↑ Scapagnini U, Matera M (November 1997). "Effects of pivagabine on psychophysical performance and behavioural response in experimental models of stress". Arzneimittel-Forschung. 47 (11A): 1310–4. PMID 9450154.
- ↑ Esposito G, Luparini MR (November 1997). "Pivagabine: a novel psychoactive drug". Arzneimittelforschung. 47 (11A): 1306–9. PMID 9450153.
- ↑ Gerra G, Zaimovic A, Giusti F; et al. (July 2001). "Pivagabine effects on neuroendocrine responses to experimentally-induced psychological stress in humans". Behavioural Brain Research. 122 (1): 93–101. doi:10.1016/S0166-4328(01)00177-2. PMID 11287080.
- ↑ Serra M, Concas A, Mostallino MC; et al. (April 1999). "Antagonism by pivagabine of stress-induced changes in GABAA receptor function and corticotropin-releasing factor concentrations in rat brain". Psychoneuroendocrinology. 24 (3): 269–84. doi:10.1016/S0306-4530(98)00049-3. PMID 10101733.
- Pages with script errors
- CS1 maint: Multiple names: authors list
- CS1 maint: Explicit use of et al.
- All articles with unsourced statements
- Articles with unsourced statements from May 2012
- Articles with invalid date parameter in template
- Chemical articles with multiple compound IDs
- Multiple chemicals in an infobox that need indexing
- Articles without InChI source
- Articles without EBI source
- Chemical articles with unknown parameter in Chembox
- ECHA InfoCard ID from Wikidata
- Articles containing unverified chemical infoboxes
- Antidepressants
- Amides
- Drug