Perkin reaction
The Perkin reaction is an organic reaction developed by William Henry Perkin that can be used to make cinnamic acids by the aldol condensation of aromatic aldehydes and acid anhydrides in the presence of an alkali salt of the acid.[1][2][3][4][5]
Erlenmeyer azlactone synthesis
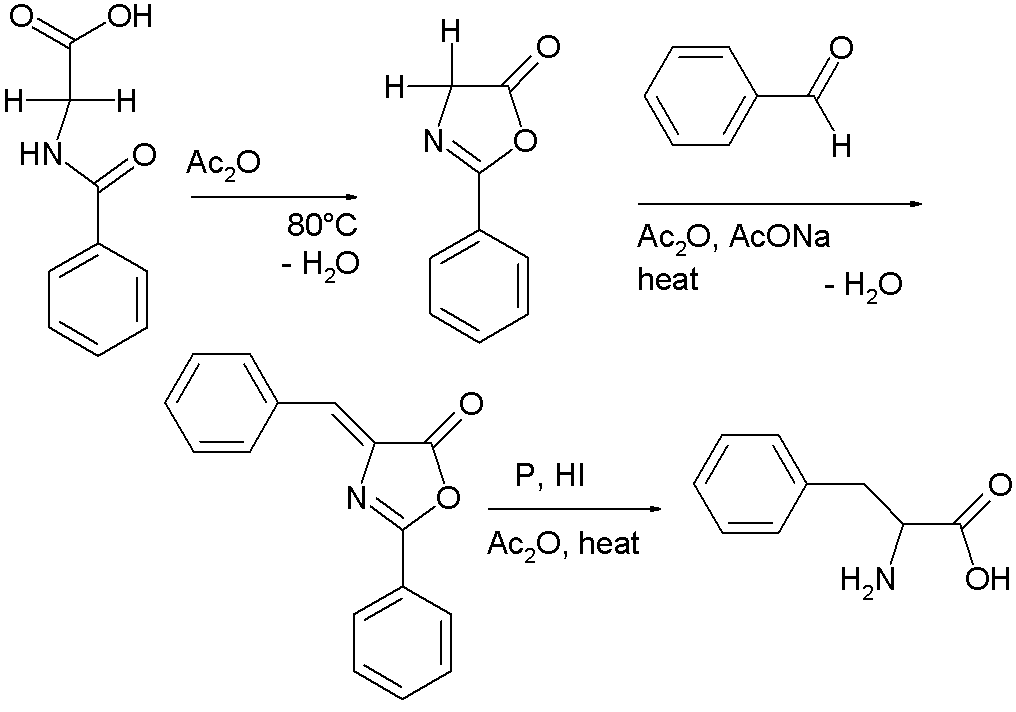
A variation is called the Erlenmeyer azlactone synthesis named after Friedrich Gustav Carl Emil Erlenmeyer [6]. In this reaction sequence hippuric acid [7] self-condenses with cyclization in the presence of acetic anhydride to 2-phenyl-oxazolone [8]. This intermediate also has two acidic protons and reacts with benzaldehyde, acetic anhydride and sodium acetate to a so-called azlactone. This compound on reduction gives access to phenylalanine [9] in the Erlenmeyer-Plochl azlactone and amino acid synthesis [10]
References
- ↑ Perkin, W. H.; J. Chem. Soc. 1868, 21, 53, 181.
- ↑ Perkin, W. H.; J. Chem. Soc. 1877, 31, 388.
- ↑ Johnson, J. R.; Org. React. 1942, 1, 210.
- ↑ House, H. O. Modern Synthetic Reactions (W. A. Benjamin, Menlo Park, California, 2nd ed, 1972) pp 660-663.
- ↑ Rosen, T.; Comp. Org. Syn. 1991, 2, 395-408.
- ↑ Erlenmeyer, Ann. 275, 3 (1893).
- ↑ Template:OrgSynth
- ↑ Template:OrgSynth
- ↑ Template:OrgSynth
- ↑ Plöchl, Ber. 17, 1623 (1884); Erlenmeyer, Ann. 275, 15 (1893);