Pertuzumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Stefano Giannoni [2];Aparna Vuppala, M.B.B.S. [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: CARDIOMYOPATHY AND EMBRYO-FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
Cardiomyopathy pertuzumab administration can result in subclinical and clinical cardiac failure. Evaluate left ventricular function in all patients prior to and during treatment with pertuzumab. Discontinue pertuzumab treatment for a confirmed clinically significant decrease in left ventricular function.
Embryo-Fetal Toxicity Exposure to pertuzumab can result in embryo-fetal death and birth defects. Studies in animals have resulted in oligohydramnios, delayed renal development, and death. Advise patients of these risks and the need for effective contraception |
Overview
Pertuzumab is a monoclonal antibody that is FDA approved for the treatment of metastatic breast cancer and as a neoadjuvant treatment of breast cancer. There is a Black Box Warning for this drug as shown here. Common adverse reactions include diarrhea, alopecia, neutropenia, nausea, vomiting, fatigue, rash, and peripheral neuropathy in combination with trastuzumab and docetaxel.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Metastatic Breast Cancer
- Indicated for use in combination with trastuzumab and docetaxel for the treatment of patients with HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease.
- Recommended initial dose of docetaxel is 75 mg/m2 administered as an intravenous infusion.
- The dose may be escalated to 100 mg/m2 administered every 3 weeks if the initial dose is well tolerated
Neoadjuvant Treatment of Breast Cancer
- Pertuzumab is indicated for use in combination with trastuzumab and docetaxel for the neoadjuvant treatment of patients with HER2-positive, locally advanced, inflammatory, or early stage breast cancer (either greater than 2 cm in diameter or node positive) as part of a complete treatment regimen for early breast cancer. This indication is based on demonstration of an improvement in pathological complete response rate. No data are available demonstrating improvement in event-free survival or overall survival.
- Pertuzumab should be administered every 3 weeks for 3 to 6 cycles as part of one of the following treatment regimens for early breast cancer:
- Four preoperative cycles of pertuzumab in combination with trastuzumab and docetaxel followed by 3 postoperative cycles of fluorouracil, epirubicin, and cyclophosphamide (FEC) as given in Study 2
- Three preoperative cycles of FEC alone followed by 3 preoperative cycles of pertuzumab in combination with docetaxel and trastuzumab as given in Study 3
- Six preoperative cycles of pertuzumab in combination with docetaxel, carboplatin, and trastuzumab (TCH) (escalation of docetaxel above 75 mg/m2 is not recommended) as given in Study 3
- Following surgery, patients should continue to receive trastuzumab to complete 1 year of treatment. There is insufficient evidence to recommend continued use of pertuzumab for greater than 6 cycles for early breast cancer. There is insufficient evidence to recommend concomitant administration of an anthracycline with pertuzumab, and there are no safety data to support sequential use of doxorubicin with pertuzumab.
Recommended Doses and Schedules
- The initial dose of pertuzumab is 840 mg administered as a 60-minute intravenous infusion, followed every 3 weeks by a dose of 420 mg administered as an intravenous infusion over 30 to 60 minutes.
- When administered with pertuzumab, the recommended initial dose of trastuzumab is 8 mg/kg administered as a 90-minute intravenous infusion, followed every 3 weeks by a dose of 6 mg/kg administered as an intravenous infusion over 30 to 90 minutes.
- Pertuzumab, trastuzumab, and docetaxel should be administered sequentially. Pertuzumab and trastuzumab can be given in any order. Docetaxel should be administered after pertuzumab and trastuzumab. An observation period of 30 to 60 minutes is recommended after each pertuzumab infusion and before commencement of any subsequent infusion of trastuzumab or docetaxel.
Dose Modification
- For delayed or missed doses, if the time between two sequential infusions is less than 6 weeks, the 420 mg dose of pertuzumab should be administered. Do not wait until the next planned dose. If the time between two sequential infusions is 6 weeks or more, the initial dose of 840 mg pertuzumab should be re-administered as a 60-minute intravenous infusion followed every 3 weeks thereafter by a dose of 420 mg administered as an intravenous infusion over 30 to 60 minutes.
- Pertuzumab should be discontinued if trastuzumab treatment is discontinued.
- Dose reductions are not recommended for pertuzumab.
- For docetaxel dose modifications, see relevant prescribing information.
Left Ventricular Ejection Fraction (LVEF):
- Withhold pertuzumab and trastuzumab dosing for at least 3 weeks for either:
- Pertuzumab may be resumed if the LVEF has recovered to greater than 49% or to 45% to 49% associated with less than a 10% absolute decrease below pretreatment values.
- If after a repeat assessment within approximately 3 weeks, the LVEF has not improved, or has declined further, pertuzumab and trastuzumab should be discontinued, unless the benefits for the individual patient are deemed to outweigh the risks.
Infusion-Related Reactions
- The infusion rate of pertuzumab may be slowed or interrupted if the patient develops an infusion-related reaction.
Hypersensitivity Reactions/Anaphylaxis
- The infusion should be discontinued immediately if the patient experiences a serious hypersensitivity reaction
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pertuzumab in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pertuzumab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Pertuzumab FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pertuzumab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pertuzumab in pediatric patients.
Contraindications
- Pertuzumab is contraindicated in patients with known hypersensitivity to pertuzumab or to any of its excipients.
Warnings
|
WARNING: CARDIOMYOPATHY AND EMBRYO-FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
Cardiomyopathy pertuzumab administration can result in subclinical and clinical cardiac failure. Evaluate left ventricular function in all patients prior to and during treatment with pertuzumab. Discontinue pertuzumab treatment for a confirmed clinically significant decrease in left ventricular function.
Embryo-Fetal Toxicity Exposure to pertuzumab can result in embryo-fetal death and birth defects. Studies in animals have resulted in oligohydramnios, delayed renal development, and death. Advise patients of these risks and the need for effective contraception |
Embryo-Fetal Toxicity
- Pertuzumab can cause fetal harm when administered to a pregnant woman.
- Treatment of pregnant cynomolgus monkeys with pertuzumab resulted in oligohydramnios, delayed fetal kidney development, and embryo-fetal death.
- If pertuzumab is administered during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to a fetus.
- Verify pregnancy status prior to the initiation of pertuzumab.
- Advise patients of the risks of embryo-fetal death and birth defects and the need for contraception during and after treatment.
- Advise patients to contact their healthcare provider immediately if they suspect they may be pregnant.
- Monitor patients who become pregnant during pertuzumab therapy for oligohydramnios. If oligohydramnios occurs, perform fetal testing that is appropriate for gestational age and consistent with community standards of care.
- The efficacy of intravenous hydration in the management of oligohydramnios due to pertuzumab exposure is not known.
Left Ventricular Dysfunction
- Decreases in LVEF have been reported with drugs that block HER2 activity, including pertuzumab.
- In Study 1, for patients with MBC, pertuzumab in combination with trastuzumab and docetaxel was not associated with increases in the incidence of symptomatic left ventricular systolic dysfunction (LVSD) or decreases in LVEF compared with placebo in combination with trastuzumab and docetaxel. Left ventricular dysfunction occurred in 4.4% of patients in the pertuzumab-treated group and 8.3% of patients in the placebo-treated group. Symptomatic left ventricular systolic dysfunction (congestive heart failure) occurred in 1.0% of patients in the pertuzumab-treated group and 1.8% of patients in the placebo-treated group.
- Patients who have received prior anthracyclines or prior radiotherapy to the chest area may be at higher risk of decreased LVEF.
- In patients receiving neoadjuvant treatment in Study 2, the incidence of LVSD was higher in the pertuzumab-treated groups compared to the trastuzumab- and docetaxel-treated group.
- An increased incidence of LVEF declines was observed in patients treated with pertuzumab in combination with trastuzumab and docetaxel. In the overall treatment period, LVEF decline > 10% and a drop to less than 50% occurred in 1.9% of patients treated with neoadjuvant trastuzumab and docetaxel as compared to 8.4% of patients treated with neoadjuvant pertuzumab in combination with trastuzumab and docetaxel.
- Symptomatic LVSD occurred in 0.9% of patients treated with neoadjuvant pertuzumab in combination with trastuzumab and no patients in the other 3 arms. LVEF recovered to ≥ 50% in all patients.
- In patients receiving neoadjuvant pertuzumab in Study 3, in the overall treatment period, LVEF decline > 10% and a drop to less than 50% occurred in 6.9% of patients treated with pertuzumab plus trastuzumab and FEC followed by pertuzumab plus trastuzumab and docetaxel, 16.0% of patients treated with pertuzumab plus trastuzumab and docetaxel following FEC, and 10.5% of patients treated with pertuzumab in combination with TCH.
- Symptomatic LVSD occurred in 4.0% of patients treated with pertuzumab plus trastuzumab and docetaxel following FEC, 1.3% of patients treated with pertuzumab in combination with TCH, and none of the patients treated with pertuzumab plus trastuzumab and FEC followed by pertuzumab plus trastuzumab and docetaxel.
- LVEF recovered to ≥ 50% in all but one patient.
- Pertuzumab has not been studied in patients with a pretreatment LVEF value of ≤ 50%, a prior history of CHF, decreases in LVEF to < 50% during prior trastuzumab therapy, or conditions that could impair left ventricular function such as uncontrolled hypertension, recent myocardial infarction, serious cardiac arrhythmia requiring treatment or a cumulative prior anthracycline exposure to > 360 mg/m2 of doxorubicin or its equivalent.
- Assess LVEF prior to initiation of pertuzumab and at regular intervals (e.g., every three months in the metastatic setting and every six weeks in the neoadjuvant setting) during treatment to ensure that LVEF is within the institution's normal limits. If LVEF is < 45%, or is 45% to 49% with a 10% or greater absolute decrease below the pretreatment value, withhold pertuzumab and trastuzumab and repeat LVEF assessment within approximately 3 weeks.
- Discontinue pertuzumab and trastuzumab if the LVEF has not improved or has declined further, unless the benefits for the individual patient outweigh the risks.
Infusion-Related Reactions
- Pertuzumab has been associated with infusion reactions.
- An infusion reactions was defined in Study 1 as any event described as hypersensitivity, anaphylactic reaction, acute infusion reaction, or cytokine release syndrome occurring during an infusion or on the same day as the infusion.
- The initial dose of pertuzumab was given the day before trastuzumab and docetaxel to allow for the examination of pertuzumab-associated reactions.
- On the first day, when only pertuzumab was administered, the overall frequency of infusion reactions was 13.0% in the pertuzumab-treated group and 9.8% in the placebo-treated group. Less than 1% were Grade 3 or 4. The most common infusion reactions (≥ 1.0%) were pyrexia, chills, fatigue, headache, asthenia, hypersensitivity, and vomiting.
- During the second cycle when all drugs were administered on the same day, the most common infusion reactions in the pertuzumab-treated group (≥ 1.0%) were fatigue, dysgeusia, hypersensitivity, myalgia, and vomiting.
- In Study 2 and Study 3, pertuzumab was administered on the same day as the other study treatment drugs.
- Infusion reactions were consistent with those observed in Study 1, with a majority of reactions being National Cancer Institute - Common Terminology Criteria for Adverse Events (NCI - CTCAE v3.0) Grade 1 – 2.
- Observe patients closely for 60 minutes after the first infusion and for 30 minutes after subsequent infusions of pertuzumab.
- If a significant infusion-related reaction occurs, slow or interrupt the infusion, and administer appropriate medical therapies.
- Monitor patients carefully until complete resolution of signs and symptoms.
- Consider permanent discontinuation in patients with severe infusion reactions.
Hypersensitivity Reactions/Anaphylaxis
- In Study 1, the overall frequency of hypersensitivity/anaphylaxis reactions was 10.8% in the pertuzumab-treated group and 9.1% in the placebo-treated group. The incidence of Grade 3 – 4 hypersensitivity/anaphylaxis reactions was 2.0% in the pertuzumab-treated group and 2.5% in the placebo-treated group according to NCI - CTCAE v3.0.
- Overall, 4 patients in pertuzumab-treated group and 2 patients in the placebo-treated group experienced anaphylaxis.
- In Study 2 and Study 3, hypersensitivity/anaphylaxis events were consistent with those observed in Study 1. In Study 2, two patients in the pertuzumab- and docetaxel-treated group experienced anaphylaxis. In Study 3, the overall frequency of hypersensitivity/anaphylaxis was highest in the pertuzumab plus TCH treated group (13.2%), of which 2.6% were NCI-CTCAE (version 3) Grade 3 – 4.
- Patients should be observed closely for hypersensitivity reactions. Severe hypersensitivity, including anaphylaxis, has been observed in clinical trials with treatment of pertuzumab. Medications to treat such reactions, as well as emergency equipment, should be available for immediate use.
- Pertuzumab is contraindicated in patients with known hypersensitivity to pertuzumab or to any of its excipients.
HER2 Testing
- Detection of HER2 protein overexpression is necessary for selection of patients appropriate for pertuzumab therapy because these are the only patients studied and for whom benefit has been shown.
- Patients with breast cancer were required to have evidence of HER2 overexpression defined as 3+ IHC or FISH amplification ratio ≥ 2.0 in the clinical studies.
- Only limited data were available for patients whose breast cancer was positive by FISH, but did not demonstrate protein overexpression by IHC.
- Assessment of HER2 status should be performed by laboratories using FDA-approved tests with demonstrated proficiency in the specific technology being utilized.
- Improper assay performance, including use of sub-optimally fixed tissue, failure to utilize specified reagents, deviation from specific assay instructions, and failure to include appropriate controls for assay validation, can lead to unreliable results.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Metastatic Breast Cancer (MBC)
The adverse reactions described in TABLE 1 were identified in 804 patients with HER2-positive metastatic breast cancer treated in Study 1. Patients were randomized to receive either pertuzumab in combination with trastuzumab and docetaxel or placebo in combination with trastuzumab and docetaxel.
- The median duration of study treatment was 18.1 months for patients in the pertuzumab-treated group and 11.8 months for patients in the placebo-treated group. No dose adjustment was permitted for pertuzumab or trastuzumab. The rates of adverse events resulting in permanent discontinuation of all study therapy were 6.1% for patients in the pertuzumab-treated group and 5.3% for patients in the placebo-treated group. Adverse events led to discontinuation of docetaxel alone in 23.6% of patients in the pertuzumab-treated group and 23.2% of patients in the placebo-treated group. TABLE 1 reports the adverse reactions that occurred in at least 10% of patients in the pertuzumab-treated group. The safety profile of pertuzumab remained unchanged with an additional year of follow-up (median total follow-up of 30 months) in Study 1.
The most common adverse reactions (> 30%) seen with pertuzumab in combination with trastuzumab and docetaxel were diarrhea, alopecia, neutropenia, nausea, fatigue, rash, and peripheral neuropathy.
- The most common NCI - CTCAE v3.0 Grade 3 – 4 adverse reactions (> 2%) were neutropenia, febrile neutropenia, leukopenia, diarrhea, peripheral neuropathy, anemia, asthenia, and fatigue.
- An increased incidence of febrile neutropenia was observed for Asian patients in both treatment arms compared with patients of other races and from other geographic regions.
- Among Asian patients, the incidence of febrile neutropenia was higher in the pertuzumab-treated group (26%) compared with the placebo-treated group (12%).

The following clinically relevant adverse reactions were reported in < 10% of patients in the pertuzumab-treated group in Study 1:
Skin and subcutaneous tissue disorders: Paronychia (7.1% in the pertuzumab-treated group vs. 3.5% in the placebo-treated group)
Respiratory, thoracic and mediastinal disorders: Pleural effusion (5.2% in the pertuzumab-treated group vs. 5.8% in the placebo-treated group)
Cardiac disorders: Left ventricular dysfunction (4.4% in the pertuzumab-treated group vs. 8.3% in the placebo-treated group) including symptomatic left ventricular systolic dysfunction (CHF) (1.0% in the pertuzumab-treated group vs. 1.8% in the placebo-treated group)
Immune system disorders: Hypersensitivity (10.1% in the pertuzumab-treated group vs. 8.6% in placebo-treated group)
Adverse Reactions Reported in Patients Receiving pertuzumab and Trastuzumab after Discontinuation of Docetaxel
In Study 1, adverse reactions were reported less frequently after discontinuation of docetaxel treatment. All adverse reactions in the pertuzumab and trastuzumab treatment group occurred in < 10% of patients with the exception of diarrhea (19.1%), upper respiratory tract infection (12.8%), rash (11.7%), headache (11.4%), and fatigue (11.1%).
Neoadjuvant Treatment of Breast Cancer (Study 2) In Study 2, the most common adverse reactions seen with pertuzumab in combination with trastuzumab and docetaxel administered for 4 cycles were similar to those seen in the pertuzumab-treated group in Study 1.
- The most common adverse reactions (> 30%) were alopecia, neutropenia, diarrhea, and nausea.
- The most common NCI – CTCAE v3.0 Grade 3 – 4 adverse reactions (> 2%) were neutropenia, febrile neutropenia, leukopenia, and diarrhea.
- In this group, one patient permanently discontinued neoadjuvant treatment due to an adverse event.
- TABLE 2 reports the adverse reactions that occurred in patients who received neoadjuvant treatment with pertuzumab for breast cancer in Study 2.

- The following adverse reactions were reported in < 10% of patients receiving neoadjuvant treatment and occurred more frequently in pertuzumab-treated groups in Study 2: (Ptz=pertuzumab; T=trastuzumab; D=docetaxel)
Blood and lymphatic system disorders:
- Anemia (6.5% in the T+D arm, 2.8% in the Ptz+T+D arm, 4.6% in the Ptz+T arm and 8.5% in the Ptz+D arm)
- Febrile neutropenia (6.5% in the T+D arm, 8.4% in the Ptz+T+D arm, 0.0% in the Ptz+T arm and 7.4% in the Ptz+D arm)
Immune system disorders:
- Hypersensitivity (1.9% in the T+D arm, 5.6% in the Ptz+T+D arm, 5.6% in the Ptz+T arm and 5.3% in the Ptz+D arm)
Nervous system disorders:
- Dizziness (3.7% in the T+D arm, 2.8% in the Ptz+T+D arm, 5.6% in the Ptz+T arm and 3.2% in the Ptz+D arm)
Infections and infestations:
- Upper respiratory tract infection (2.8% in the T+D arm, 4.7% in the Ptz+T+D arm, 1.9% in the Ptz+T arm and 7.4% in the Ptz+D arm)
Respiratory, thoracic and mediastinal disorders:
- Dyspnea (3.7% in the T+D arm, 4.7% in the Ptz+T+D arm, 2.8% in the Ptz+T arm and 2.1% in the Ptz+D arm)
Cardiac disorders:
- Left ventricular dysfunction (0.9% in the T+D arm, 2.8% in the Ptz+T+D arm, 0.0% in the Ptz+T arm, and 1.1% in the Ptz+D arm) including symptomatic left ventricular dysfunction (CHF) (0.9% in the Ptz+T arm and 0.0% in the T+D arm, Ptz+T+D arm, and Ptz+D arm)
Eye disorders:
- Lacrimation increased (1.9% in the T+D arm, 3.7% in the Ptz+T+D arm, 0.9% in the Ptz+T arm, and 4.3% in the Ptz+D arm)
Neoadjuvant Treatment of Breast Cancer (Study 3)
- In Study 3, when pertuzumab was administered in combination with trastuzumab and docetaxel for 3 cycles following 3 cycles of FEC, the most common adverse reactions (> 30%) were diarrhea, nausea, alopecia, neutropenia, vomiting, and fatigue.
- The most common NCI-CTCAE (version 3) Grade 3 – 4 adverse reactions (> 2%) were neutropenia, leukopenia, febrile neutropenia, diarrhea, left ventricular dysfunction, anemia, dyspnea, nausea, and vomiting.
Similarly, when pertuzumab was administered in combination with docetaxel, carboplatin, and trastuzumab (TCH) for 6 cycles, the most common adverse reactions (> 30%) were diarrhea, alopecia, neutropenia, nausea, fatigue, vomiting, anemia, and thrombocytopenia.
- The most common NCI-CTCAE (version 3) Grade 3 – 4 adverse reactions (> 2%) were neutropenia, febrile neutropenia, anemia, leukopenia, diarrhea, thrombocytopenia, vomiting, fatigue, ALT increased, hypokalemia, and hypersensitivity.
- The rates of adverse events resulting in permanent discontinuation of any component of neoadjuvant treatment were 6.7% for patients receiving pertuzumab in combination with trastuzumab and docetaxel following FEC and 7.9% for patients receiving pertuzumab in combination with TCH.
- TABLE 3 reports the adverse reactions that occurred in patients who received neoadjuvant treatment with pertuzumab for breast cancer in Study 3.

The following selected adverse reactions were reported in < 10% of patients receiving neoadjuvant treatment in Study 3: (Ptz=pertuzumab; T=trastuzumab; D=docetaxel; FEC= fluorouracil, epirubicin, and cyclophosphamide; TCH=docetaxel, carboplatin, and trastuzumab)
Skin and subcutaneous tissue disorders: Nail disorder (9.7% in the Ptz+T+FEC/Ptz+T+D arm, 6.7% in the FEC/Ptz+T+D arm, and 9.2% in the Ptz+TCH arm), Paronychia (0% in the Ptz+T+FEC/Ptz+T+D and 1.3% in both the FEC/Ptz+T+D and Ptz+TCH arms), Pruritis (2.8% in the Ptz+T+FEC/Ptz+T+D arm, 4.0% in the FEC/Ptz+T+D arm, and 3.9% in the Ptz+TCH arm)
Infections and infestations:
- Upper respiratory tract infection (8.3% in the Ptz+T+FEC/Ptz+T+D arm, 4.0% in the FEC/Ptz+T+D arm, and 2.6% in the Ptz+TCH arm), Nasopharyngitis (6.9% in the Ptz+T+FEC/Ptz+T+D arm, 6.7% in the FEC/Ptz+T+D arm, and 7.9% in the Ptz+TCH arm)
Respiratory, thoracic, and mediastinal disorders:
- Pleural effusion (1.4% in the Ptz+T+FEC/Ptz+T+D arm and 0% in the FEC/Ptz+T+D and Ptz+TCH arm)
Cardiac disorders:
- Left ventricular dysfunction (5.6% in the Ptz+T+FEC/PTZ+T+D arm, 4.0% in the FEC/Ptz+T+D arm, and 2.6% in the Ptz+TCH arm) including symptomatic left ventricular systolic dysfunction (CHF) (2.7% in the FEC/Ptz+T+D arm and 0% in the Ptz+T+FEC/Ptz+T+D and Ptz+TCH arms)
Immunogenicity
- As with all therapeutic proteins, there is the potential for an immune response to pertuzumab.
- Patients in Study 1 were tested at multiple time-points for antibodies to pertuzumab.
- Approximately 2.8% (11/386) of patients in the pertuzumab-treated group and 6.2% (23/372) of patients in the placebo-treated group tested positive for anti-pertuzumab antibodies.
- Of these 34 patients, none experienced anaphylactic/hypersensitivity reactions that were clearly related to the anti-therapeutic antibodies (ATA).
- The presence of pertuzumab in patient serum at the levels expected at the time of ATA sampling can interfere with the ability of this assay to detect anti-pertuzumab antibodies.
- In addition, the assay may be detecting antibodies to trastuzumab.
- As a result, data may not accurately reflect the true incidence of anti-pertuzumab antibody development.
- Immunogenicity data are highly dependent on the sensitivity and specificity of the test methods used.
- Additionally, the observed incidence of a positive result in a test method may be influenced by several factors, including sample handling, timing of sample collection, drug interference, concomitant medication, and the underlying disease. For these reasons, comparison of the incidence of antibodies to pertuzumab with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
There is limited information regarding Pertuzumab Postmarketing Experience in the drug label.
Drug Interactions
No drug-drug interactions were observed between pertuzumab and trastuzumab, or between pertuzumab and docetaxel.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies of pertuzumab in pregnant women. Based on findings in animal studies, pertuzumab can cause fetal harm when administered to a pregnant woman. The effects of pertuzumab are likely to be present during all trimesters of pregnancy. Pertuzumab administered to pregnant cynomolgus monkeys resulted in oligohydramnios, delayed fetal kidney development, and embryo-fetal deaths at clinically relevant exposures of 2.5 to 20-fold greater than the recommended human dose, based on Cmax. If pertuzumab is administered during pregnancy, or if a patient becomes pregnant while receiving pertuzumab, the patient should be apprised of the potential hazard to the fetus.
Animal Data
- Reproductive toxicology studies have been conducted in cynomolgus monkeys. Pregnant monkeys were treated on Gestational Day (GD)19 with loading doses of 30 to 150 mg/kg pertuzumab, followed by bi-weekly doses of 10 to 100 mg/kg. These dose levels resulted in clinically relevant exposures of 2.5 to 20-fold greater than the recommended human dose, based on Cmax. Intravenous administration of pertuzumab from GD19 through GD50 (period of organogenesis) was embryotoxic, with dose-dependent increases in embryo-fetal death between GD25 to GD70. The incidences of embryo-fetal loss were 33, 50, and 85% for dams treated with bi-weekly pertuzumab doses of 10, 30, and 100 mg/kg, respectively (2.5 to 20-fold greater than the recommended human dose, based on Cmax). At Caesarean section on GD100, oligohydramnios, decreased relative lung and kidney weights, and microscopic evidence of renal hypoplasia consistent with delayed renal development were identified in all pertuzumab dose groups. Pertuzumab exposure was reported in offspring from all treated groups, at levels of 29% to 40% of maternal serum levels at GD100.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pertuzumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pertuzumab during labor and delivery.
Nursing Mothers
- It is not known whether pertuzumab is excreted in human milk, but human IgG is excreted in human milk. Because many drugs are secreted in human milk and because of the potential for serious adverse reactions in nursing infants from pertuzumab, a decision should be made whether to discontinue nursing, or discontinue drug, taking into account the elimination half-life of pertuzumab and the importance of the drug to the mother
Pediatric Use
- The safety and effectiveness of pertuzumab have not been established in pediatric patients.
Geriatic Use
- Of 402 patients who received pertuzumab in Study 1, 60 patients (15%) were ≥ 65 years of age and 5 patients (1%) were ≥ 75 years of age. No overall differences in efficacy and safety of pertuzumab were observed between these patients and younger patients.
Based on a population pharmacokinetic analysis, no significant difference was observed in the pharmacokinetics of pertuzumab between patients < 65 years (n=306) and patients ≥ 65 years (n=175).
Gender
There is no FDA guidance on the use of Pertuzumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pertuzumab with respect to specific racial populations.
Renal Impairment
Dose adjustments of pertuzumab are not needed in patients with mild (creatinine clearance [[[CLcr]]] 60 to 90 mL/min) or moderate (CLcr 30 to 60 mL/min) renal impairment. No dose adjustment can be recommended for patients with severe renal impairment (CLcr less than 30 mL/min) because of the limited pharmacokinetic data available
Hepatic Impairment
- No clinical studies have been conducted to evaluate the effect of hepatic impairment on the pharmacokinetics of pertuzumab.
Females of Reproductive Potential and Males
- Pertuzumab can cause embryo-fetal harm when administered during pregnancy. Counsel patients regarding pregnancy prevention and planning. *Advise females of reproductive potential to use effective contraception while receiving pertuzumab and for 6 months following the last dose of pertuzumab.
Immunocompromised Patients
There is no FDA guidance one the use of Pertuzumab in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
- Infusion-Related Reactions: Monitor for signs and symptoms. If a significant infusion-associated reaction occurs, slow or interrupt the infusion and administer appropriate medical therapies.
- Hypersensitivity Reactions/Anaphylaxis: Monitor for signs and symptoms
- Monitor patients who become pregnant during pertuzumab therapy for oligohydramnios.
IV Compatibility
Preparation for Administration
Administer as an intravenous infusion only. Do not administer as an intravenous push or bolus. Do not mix pertuzumab with other drugs.
Preparation
Prepare the solution for infusion, using aseptic technique, as follows:
- Parenteral drug products should be inspected visually for particulates and discoloration prior to administration.
- Withdraw the appropriate volume of pertuzumab solution from the vial(s).
- Dilute into a 250 mL 0.9% sodium chloride PVC or non-PVC polyolefin infusion bag.
- Mix diluted solution by gentle inversion. Do not shake.
- Administer immediately once prepared.
- If the diluted infusion solution is not used immediately, it can be stored at 2°C to 8°C for up to 24 hours.
- Dilute with 0.9% Sodium Chloride injection only. Do not use dextrose (5%) solution.
Overdosage
No drug overdoses have been reported with pertuzumab to date.
Pharmacology
| |
Pertuzumab?
| |
| Therapeutic monoclonal antibody | |
| Source | zu/o |
| Target | HER2 |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
D(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Intravenous |
Mechanism of Action
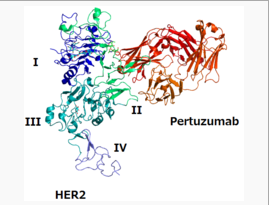
Pertuzumab targets the extracellular dimerization domain (Subdomain II) of the human epidermal growth factor receptor 2 protein (HER2) and, thereby, blocks ligand-dependent heterodimerization of HER2 with other HER family members, including EGFR, HER3, and HER4. As a result, pertuzumab inhibits ligand-initiated intracellular signaling through two major signal pathways, mitogen-activated protein (MAP) kinase, and phosphoinositide 3-kinase (PI3K). Inhibition of these signaling pathways can result in cell growth arrest and apoptosis, respectively. In addition, pertuzumab mediates antibody-dependent cell-mediated cytotoxicity (ADCC).
While pertuzumab alone inhibited the proliferation of human tumor cells, the combination of pertuzumab and trastuzumab augmented anti-tumor activity in HER2-overexpressing xenograft models.
Structure
There is limited information regarding Pertuzumab Structure in the drug label.
Pharmacodynamics
Cardiac Electrophysiology
The effect of pertuzumab with an initial dose of 840 mg followed by a maintenance dose of 420 mg every three weeks on QTc interval was evaluated in a subgroup of 20 patients with HER2-positive breast cancer in Study 1. No large changes in the mean QT interval (i.e., greater than 20 ms) from placebo based on Fridericia correction method were detected in the trial. A small increase in the mean QTc interval (i.e., less than 10 ms) cannot be excluded because of the limitations of the trial design.
Pharmacokinetics
Pertuzumab demonstrated linear pharmacokinetics at a dose range of 2 – 25 mg/kg. Based on a population PK analysis that included 481 patients, the median clearance (CL) of pertuzumab was 0.24 L/day and the median half-life was 18 days. With an initial dose of 840 mg followed by a maintenance dose of 420 mg every three weeks thereafter, the steady-state concentration of pertuzumab was reached after the first maintenance dose. The population PK analysis suggested no PK differences based on age, gender, ethnicity (Japanese vs. non-Japanese), or disease status (neoadjuvant versus metastatic setting). Baseline serum albumin level and lean body weight as covariates only exerted a minor influence on PK parameters. Therefore, no dose adjustments based on body weight or baseline albumin level are needed. No drug-drug interactions were observed between pertuzumab and trastuzumab, or between pertuzumab and docetaxel in a sub-study of 37 patients in Study 1. No dedicated renal impairment trial for pertuzumab has been conducted. Based on the results of the population pharmacokinetic analysis, pertuzumab exposure in patients with mild (CLcr 60 to 90 mL/min, n=200) and moderate renal impairment (CLcr 30 to 60 mL/min, n=71) were similar to those in patients with normal renal function (CLcr greater than 90 mL/min, n=200). No relationship between CLcr and pertuzumab exposure was observed over the range of observed CLcr (27 to 244 mL/min).
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of pertuzumab. Studies have not been performed to evaluate the mutagenic potential of pertuzumab. No specific fertility studies in animals have been performed to evaluate the effect of pertuzumab. No adverse effects on male and female reproductive organs were observed in repeat-dose toxicity studies of up to six months duration in cynomolgus monkeys.
Clinical Studies
Metastatic Breast Cancer
Study 1 was a multicenter, double-blind, placebo-controlled trial of 808 patients with HER2-positive metastatic breast cancer. HER2 overexpression was defined as a score of 3+ IHC or FISH amplification ratio of 2.0 or greater as determined by a central laboratory. Patients were randomly allocated 1:1 to receive placebo plus trastuzumab and docetaxel or pertuzumab plus trastuzumab and docetaxel. Randomization was stratified by prior treatment (prior or no prior adjuvant/neoadjuvant anti-HER2 therapy or chemotherapy) and geographic region (Europe, North America, South America, and Asia). Patients with prior adjuvant or neoadjuvant therapy were required to have a disease-free interval of greater than 12 months before trial enrollment. Pertuzumab was given intravenously at an initial dose of 840 mg, followed by 420 mg every 3 weeks thereafter. Trastuzumab was given intravenously at an initial dose of 8 mg/kg, followed by 6 mg/kg every 3 weeks thereafter. Patients were treated with pertuzumab and trastuzumab until progression of disease, withdrawal of consent, or unacceptable toxicity. Docetaxel was given as an initial dose of 75 mg/m2 by intravenous infusion every 3 weeks for at least 6 cycles. The docetaxel dose could be escalated to 100 mg/m2 at the investigator's discretion if the initial dose was well tolerated. At the time of the primary analysis, the mean number of cycles of study treatment administered was 16.2 in the placebo-treated group and 19.9 in the pertuzumab-treated group. The primary endpoint of Study 1 was progression-free survival (PFS) as assessed by an independent review facility (IRF). PFS was defined as the time from the date of randomization to the date of disease progression or death (from any cause) if the death occurred within 18 weeks of the last tumor assessment. Additional endpoints included overall survival (OS), PFS (investigator-assessed), objective response rate (ORR), and duration of response. Patient demographic and baseline characteristics were balanced between the treatment arms. The median age was 54 (range 22 to 89 years), 59% were White, 32% were Asian, and 4% were Black. All were women with the exception of 2 patients. Seventeen percent of patients were enrolled in North America, 14% in South America, 38% in Europe, and 31% in Asia. Tumor prognostic characteristics, including hormone receptor status (positive 48%, negative 50%), presence of visceral disease (78%) and non-visceral disease only (22%) were similar in the study arms. Approximately half of the patients received prior adjuvant or neoadjuvant anti-HER2 therapy or chemotherapy (placebo 47%, pertuzumab 46%). Among patients with hormone receptor positive tumors, 45% received prior adjuvant hormonal therapy and 11% received hormonal therapy for metastatic disease. Eleven percent of patients received prior adjuvant or neoadjuvant trastuzumab.
Study 1 demonstrated a statistically significant improvement in IRF-assessed PFS in the pertuzumab-treated group compared with the placebo-treated group [hazard ratio (HR)=0.62 (95% CI: 0.51, 0.75), p < 0.0001] and an increase in median PFS of 6.1 months (median PFS of 18.5 months in the pertuzumab-treated group vs. 12.4 months in the placebo-treated group) (see FIGURE 1). The results for investigator-assessed PFS were comparable to those observed for IRF-assessed PFS. Consistent results were observed across several patient subgroups including age (< 65 or ≥ 65 years), race, geographic region, prior adjuvant/neoadjuvant anti-HER2 therapy or chemotherapy (yes or no), and prior adjuvant/neoadjuvant trastuzumab (yes or no). In the subgroup of patients with hormone receptor-negative disease (n=408), the hazard ratio was 0.55 (95% CI: 0.42, 0.72). In the subgroup of patients with hormone receptor-positive disease (n=388), the hazard ratio was 0.72 (95% CI: 0.55, 0.95). In the subgroup of patients with disease limited to non-visceral metastasis (n=178), the hazard ratio was 0.96 (95% CI: 0.61, 1.52). At the time of the final PFS analysis, 165 patients had died, and more deaths had occurred in the placebo-treated group (23.6%) compared with the pertuzumab-treated group (17.2%); OS was not mature and interim OS analysis results did not meet the pre-specified stopping boundary for statistical significance. A second interim analysis of OS, conducted after an additional year of follow-up, demonstrated a statistically significant improvement in OS [HR=0.66 (95% CI: 0.52, 0.84), p=0.0008]. See TABLE 4 and FIGURE 2. OS results in patient subgroups were consistent with those observed for IRF-assessed PFS with the exception of the subgroup of patients with disease limited to non-visceral metastasis [HR=1.42 (95% CI: 0.71, 2.84)].



Neoadjuvant Treatment of Breast Cancer Study 2
- Study 2 was a multicenter, randomized trial conducted in 417 patients with operable, locally advanced, or inflammatory HER2-positive breast cancer (T2-4d) who were scheduled for neoadjuvant therapy. HER2 overexpression was defined as a score of 3+ IHC or FISH amplification ratio of 2.0 or greater as determined by a central laboratory. Patients were randomly allocated to receive 1 of 4 neoadjuvant regimens prior to surgery as follows: trastuzumab plus docetaxel, pertuzumab plus trastuzumab and docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel. Randomization was stratified by breast cancer type (operable, locally advanced, or inflammatory) and estrogen receptor (ER) or progesterone receptor (PgR) positivity.
- pertuzumab was given intravenously at an initial dose of 840 mg, followed by 420 mg every 3 weeks for 4 cycles. Trastuzumab was given intravenously at an initial dose of 8 mg/kg, followed by 6 mg/kg every 3 weeks for 4 cycles. Docetaxel was given as an initial dose of 75 mg/m2 by intravenous infusion every 3 weeks for 4 cycles. The docetaxel dose could be escalated to 100 mg/m2 at the investigator's discretion if the initial dose was well tolerated. Following surgery all patients received 3 cycles of 5-fluorouracil (600 mg/m2), epirubicin (90 mg/m2), and cyclophosphamide (600 mg/m2) (FEC) given intravenously every 3 weeks and trastuzumab administered intravenously every 3 weeks to complete 1 year of therapy. After surgery, patients in the pertuzumab plus trastuzumab arm received docetaxel every 3 weeks for 4 cycles prior to FEC.
- The primary endpoint of the study was pathological complete response (pCR) rate in the breast (ypT0/is). The FDA-preferred definition of pCR is the absence of invasive cancer in the breast and lymph nodes (ypT0/is ypN0).
- Demographics were well balanced (median age was 49 – 50 years old, the majority were Caucasian (71%) and all were female. Overall, 7% of patients had inflammatory cancer, 32% had locally advanced cancer, and 61% had operable cancer. Approximately half the patients in each treatment group had hormone receptor-positive disease (defined as ER-positive and/or PgR-positive).
- The efficacy results are summarized in TABLE 5. Statistically significant improvements in pCR rates by both the study and FDA-preferred definitions were observed in patients receiving pertuzumab plus trastuzumab and docetaxel compared to patients receiving trastuzumab plus docetaxel. The pCR rates and magnitude of improvement with pertuzumab were lower in the subgroup of patients with hormone receptor-positive tumors compared to patients with hormone receptor-negative tumors.

Study 3
- An additional phase 2 neoadjuvant study was conducted in 225 patients with HER2-positive locally advanced, operable, or inflammatory (T2-4d) breast cancer designed primarily to assess cardiac safety in which all arms included pertuzumab. HER2 overexpression was defined as a score of 3+ IHC or FISH amplification ratio of 2.0 or greater as determined by a central laboratory.
- Patients were randomly allocated to receive 1 of 3 neoadjuvant regimens prior to surgery as follows: 3 cycles of FEC followed by 3 cycles of docetaxel all in combination with pertuzumab and trastuzumab, 3 cycles of FEC alone followed by 3 cycles of docetaxel and trastuzumab in combination with pertuzumab, or 6 cycles of docetaxel, carboplatin, and trastuzumab (TCH) in combination with pertuzumab. Randomization was stratified by breast cancer type (operable, locally advanced, or inflammatory) and ER and/or PgR positivity.
- pertuzumab was given by intravenous infusion at an initial dose of 840 mg, followed by 420 mg every 3 weeks. Trastuzumab was given by intravenous infusion at an initial dose of 8 mg/kg, followed by 6 mg/kg every 3 weeks. 5-Fluorouracil (500 mg/m2), epirubicin (100 mg/m2), and cyclophosphamide (600 mg/m2) were given intravenously every 3 weeks for 3 cycles. In the pertuzumab plus trastuzumab, docetaxel, and FEC arms, docetaxel was given as an initial dose of 75 mg/m2 by intravenous infusion every 3 weeks for 3 cycles with the option to escalate to 100 mg/m2 at the investigator's discretion if the initial dose was well tolerated. However, in the pertuzumab plus TCH arm, docetaxel was given intravenously at 75 mg/m2 (no escalation was permitted) and carboplatin (AUC 6) was given intravenously every 3 weeks for 6 cycles. Following surgery all patients received trastuzumab to complete 1 year of therapy, which was administered intravenously every 3 weeks.
- Demographics were well balanced (median age was 49-50 years old, the majority were Caucasian (76%)) and all were female. Overall 6% of patients had inflammatory cancer, 25% had locally advanced cancer and 69% had operable cancer, with approximately half the patients in each treatment group having ER-positive and/or PgR-positive disease.
- The pCR (ypT0/is ypN0) rates were 56.2% (95% CI: 44.1%, 67.8%), 54.7% (95% CI: 42.7%, 66.2%), and 63.6% (95% CI: 51.9%, 74.3%) for patients treated with pertuzumab plus trastuzumab and FEC followed by pertuzumab plus trastuzumab and docetaxel, pertuzumab plus trastuzumab and docetaxel following FEC, or pertuzumab plus TCH, respectively. The pCR rates were lower in the subgroups of patients with hormone receptor-positive tumors: 41.0% (95% CI: 25.6%, 57.9%), 45.7% (95% CI: 28.8%, 63.4%), and 47.5% (95% CI: 31.5%, 63.9%) than with hormone receptor-negative tumors: 73.5% (95% CI: 55.6%, 87.1%), 62.5% (95% CI: 45.8%, 77.3%), and 81.1% (95% CI: 64.8%, 92.0%), respectively.
How Supplied
- Pertuzumab is supplied as:
- 420 mg/14 mL (30 mg/mL) single-use vial containing preservative-free solution.
- NDC 50242-145-01.
Storage
- Store vials in a refrigerator at 2°C to 8°C (36°F to 46°F) until time of use.
- Keep vial in the outer carton in order to protect from light.
- DO NOT FREEZE. DO NOT SHAKE.
Images
Drug Images
{{#ask: Page Name::Pertuzumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Pertuzumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise pregnant women and females of reproductive potential that pertuzumab exposure can result in fetal harm, including embryo-fetal death or birth defects.
- Advise females of reproductive potential to use effective contraception while receiving pertuzumab and for 6 months following the last dose of pertuzumab.
- Advise nursing mothers treated with pertuzumab to discontinue nursing or discontinue pertuzumab, taking into account the importance of the drug to the mother.
- Encourage women who are exposed to pertuzumab during pregnancy to enroll in the MotHER Pregnancy Registry by contacting 1-800-690-6720.
Precautions with Alcohol
Alcohol-Pertuzumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Pertuzumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Pertuzumab |Label Name=Pertuzumab Package.png
}}