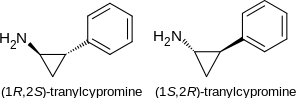
Tranylcypromine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Pratik Bahekar, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Suicidality and Antidepressant Drugs
See full prescribing information for complete Boxed Warning.
Condition Name: Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of PARNATE or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. PARNATE is not approved for use in pediatric patients. (See warning to physicians: Clinical Worsening and Suicide Risk, precautions: Information for Patients, and Precautions: Pediatric Use.)
|
Overview
Tranylcypromine is a MAOIs that is FDA approved for the {{{indicationType}}} of major depressive disorder. There is a Black Box Warning for this drug as shown here. Common adverse reactions include edema, weight gain, constipation, loss of appetite, nausea, xerostomia, asthenia, dizziness, headache, insomnia, somnolence, agitation, worsening, anxiety, impotence.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Major depressive disorder
- 30 mg/day PO, after 2 weeks, may increase by 10 mg/day every 1-3 weeks to a :*Maximum dose of 60 mg/day
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Tranylcypromine in adult patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Tranylcypromine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in children have not been established
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Tranylcypromine in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Tranylcypromine in pediatric patients.
Contraindications
- Parnate(tranylcypromine sulfate)should not be administered to any patient with a confirmed or suspected cerebrovascular defect or to any patient with cardiovascular disease or hypertension.
- Parnate(tranylcypromine sulfate)should not be used in the presence of pheochromocytoma since such tumors secrete pressor substances.
- Tranylcypromine sulfate should not be administered together or in rapid succession with other MAO inhibitors or with dibenzazepine-related entities. Hypertensive crises or severe convulsive seizures may occur in patients receiving such combinations.
- As a general rule, Parnate should not be administered in combination with any SSRI or SNRI. There have been reports of serious, sometimes fatal, reactions (including hyperthermia, rigidity, myoclonus, autonomic instability with possible rapid fluctuations of vital signs, and mental status changes that include extreme agitation progressing to delirium and coma) in patients receiving a SSRI (e.g., fluoxetine) or a SNRI (e.g., venlafaxine) in combination with a monoamine oxidase inhibitor (MAOI), and in patients who have recently discontinued a SSRI or SNRI and are then started on an MAOI.
- Parnate should not be used in combination with buspirone HCl, since several cases of elevated blood pressure have been reported in patients taking MAO inhibitors who were then given buspirone HCl.
- Parnate should not be administered in combination with sympathomimetics, including amphetamines which may be found in many herbal preparations as well as over-the-counter drugs such as cold, hay fever or weight-reducing preparations that contain vasoconstrictors.
- Do not use meperidine concomitantly with MAO inhibitors or within 2 or 3 weeks following MAOI therapy. Serious reactions have been precipitated with concomitant use, including coma, severe hypertension or hypotension, severe respiratory depression, convulsions, malignant hyperpyrexia, excitation, peripheral vascular collapse, and death.
- The combination of MAO inhibitors and dextromethorphan has been reported to cause brief episodes of psychosis or bizarre behavior.
- When excessive amounts of tyramine are consumed in conjunction with tranylcypromine, or within 2 weeks of stopping treatment, a serious and sometimes fatal hypertensive reaction may occur.
- Patients taking parnate should not undergo elective surgery requiring general anesthesia. Also, they should not be given cocaine or local anesthesia containing sympathomimetic vasoconstrictors. The possible combined hypotensive effects of parnate and spinal anesthesia should be kept in mind. Parnate should be discontinued at least 10 days prior to elective surgery.
- Parnate should not be used in combination with some central nervous system depressants such as narcotics and alcohol, or with hypotensive agents.
- Anti-parkinsonism drugs should be used with caution in patients receiving parnate since severe reactions have been reported.
- Parnate should not be used in patients with a history of liver disease or in those with abnormal liver function tests.
- Excessive use of caffeine in any form should be avoided in patients receiving parnate.
Warnings
|
Suicidality and Antidepressant Drugs
See full prescribing information for complete Boxed Warning.
Condition Name: Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of PARNATE or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. PARNATE is not approved for use in pediatric patients. (See warning to physicians: Clinical Worsening and Suicide Risk, precautions: Information for Patients, and Precautions: Pediatric Use.)
|
Hypertensive Crisis
The most important reaction associated with PARNATE is the occurrence of hypertensive crises which have sometimes been fatal.
These crises are characterized by some or all of the following symptoms: occipital headache which may radiate frontally, palpitation, neck stiffness or soreness, nausea or vomiting, sweating (sometimes with fever and sometimes with cold, clammy skin), and photophobia. Either tachycardia or bradycardia may be present, and associated constricting chest pain and dilated pupils may occur.
Intracranial bleeding, sometimes fatal in outcome, has been reported in association with the paradoxical increase in blood pressure.
In all patients taking PARNATE, blood pressure should be followed closely to detect evidence of any pressor response. It is emphasized that full reliance should not be placed on blood pressure readings, but that the patient should also be observed frequently.
Therapy should be discontinued immediately upon the occurrence of palpitation or frequent headaches during therapy with PARNATE. These signs may be prodromal of a hypertensive crisis.
Hypotension
Hypotension has been observed during therapy with parnate. Symptoms of postural hypotension are seen most commonly but not exclusively in patients with pre-existent hypertension; blood pressure usually returns rapidly to pretreatment levels upon discontinuation of the drug. At doses above 30 mg daily, postural hypotension is a major side effect and may result in syncope. Dosage increases should be made more gradually in patients showing a tendency toward hypotension at the beginning of therapy. Postural hypotension may be relieved by having the patient lie down until blood pressure returns to normal. Also, when parnate is combined with those phenothiazine derivatives or other compounds known to cause hypotension, the possibility of additive hypotensive effects should be considered. There have been reports of drug dependency in patients using doses of tranylcypromine significantly in excess of the therapeutic range. Some of these patients had a history of previous substance abuse. The following withdrawal symptoms have been reported: restlessness, anxiety, depression, confusion, hallucinations, headache, weakness, and diarrhea. Drugs which lower the seizure threshold, including MAO inhibitors, should not be used with Amipaque®*. As with other MAO inhibitors,parnate should be discontinued at least 48 hours before myelography and should not be resumed for at least 24 hours postprocedure. MAO inhibitors may have the capacity to suppress anginal pain that would otherwise serve as a warning of myocardial ischemia. The usual precautions should be observed in patients with impaired renal function since there is a possibility of cumulative effects in such patients. Older patients may suffer more morbidity than younger patients during and following an episode of hypertension or malignant hyperthermia. Older patients have less compensatory reserve to cope with any serious adverse reaction. Therefore, parnate should be used with caution in the elderly population. Although excretion of parnate is rapid, inhibition of MAO may persist up to 10 days following discontinuation. Because the influence of parnate on the convulsive threshold is variable in animal experiments, suitable precautions should be taken if epileptic patients are treated. Some MAO inhibitors have contributed to hypoglycemic episodes in diabetic patients receiving insulin or oral hypoglycemic agents. Therefore, parnate should be used with caution in diabetics using these drugs. Parnate may aggravate coexisting symptoms in depression, such as anxiety and agitation. Use parnate with caution in hyperthyroid patients because of their increased sensitivity to pressor amines. Parnate should be administered with caution to patients receiving Antabuse®†. In a single study, rats given high intraperitoneal doses of d or l isomers of tranylcypromine sulfate plus disulfiram experienced severe toxicity including convulsions and death. Additional studies in rats given high oral doses of racemic tranylcypromine sulfate (Parnate) and disulfiram produced no adverse interaction.
Adverse Reactions
Clinical Trials Experience
- Overstimulation which may include increased anxiety, agitation, and manic symptoms is usually evidence of excessive therapeutic action. Dosage should be reduced, or a phenothiazine tranquilizer should be administered concomitantly.
- Patients may experience restlessness or insomnia; may notice some weakness, drowsiness, episodes of dizziness or dry mouth; or may report nausea, diarrhea, abdominal pain, or constipation. Most of these effects can be relieved by lowering the dosage or by giving suitable concomitant medication.
- Tachycardia, significant anorexia, edema, palpitation, blurred vision, chills, and impotence have each been reported.
- Headaches without blood pressure elevation have occurred.
- Rare instances of hepatitis, skin rash, and alopecia have been reported.
- Impaired water excretion compatible with the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) has been reported.
- Tinnitus, muscle spasm, tremors, myoclonic jerks, numbness, paresthesia, urinary retention, and retarded ejaculation have been reported.
- Hematologic disorders including anemia, leukopenia, agranulocytosis, and thrombocytopenia have been reported.
Post-Introduction Reports
- The following are spontaneously reported adverse events temporally associated with use of tranylcypromine sulfate. No clear relationship between tranylcypromine sulfate and these events has been established. Localized scleroderma, flare-up of cystic acne, ataxia, confusion, disorientation, memory loss, urinary frequency, urinary incontinence, urticaria, fissuring in corner of mouth, akinesia.
Postmarketing Experience
There is limited information regarding Tranylcypromine Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Tranylcypromine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
Use of any drug in pregnancy, during lactation or in women of childbearing age requires that the potential benefits of the drug be weighed against its possible hazards to mother and child.
Animal reproductive studies show that PARNATE passes through the placental barrier into the fetus of the rat, and into the milk of the lactating dog. The absence of a harmful action of PARNATE on fertility or on postnatal development by either prenatal treatment or from the milk of treated animals has not been demonstrated. Tranylcypromine is excreted in human milk.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tranylcypromine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tranylcypromine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Tranylcypromine in women who are nursing.
Pediatric Use
Safety and effectiveness in the pediatric population have not been established (see box warning and warnings—Clinical Worsening and Suicide Risk). Anyone considering the use of Parnate in a child or adolescent must balance the potential risks with the clinical need.
Geriatic Use
There is no FDA guidance on the use of Tranylcypromine in geriatric settings.
Gender
There is no FDA guidance on the use of Tranylcypromine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tranylcypromine with respect to specific racial populations.
Renal Impairment
The usual precautions should be observed in patients with impaired renal function since there is a possibility of cumulative effects in such patients.
Hepatic Impairment
Parnate should not be used in patients with a history of liver disease or in those with abnormal liver function tests.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tranylcypromine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tranylcypromine in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Tranylcypromine Administration in the drug label.
Monitoring
There is limited information regarding Tranylcypromine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Tranylcypromine and IV administrations.
Overdosage
There is limited information regarding Tranylcypromine overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
There is limited information regarding Tranylcypromine Mechanism of Action in the drug label.
Structure
There is limited information regarding Tranylcypromine Structure in the drug label.
Pharmacodynamics
There is limited information regarding Tranylcypromine Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Tranylcypromine Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Tranylcypromine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Tranylcypromine Clinical Studies in the drug label.
How Supplied
There is limited information regarding Tranylcypromine How Supplied in the drug label.
Storage
There is limited information regarding Tranylcypromine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Tranylcypromine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Tranylcypromine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with PARNATE and should counsel them in its appropriate use. A patient Medication Guide about “Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions” is available for parnate. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document. Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking parnate.
Precautions with Alcohol
Alcohol-Tranylcypromine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Tranylcypromine Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Tranylcypromine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 1.2 Williams, David A. (2007). = R0W1ErpsQpkC&pg = PA590 "Antidepressants" Check
|chapterurl=value (help). In Foye, William O.; Lemke, Thomas L.; Williams, David A. Foye's Principles of Medicinal Chemistry. Hagerstwon, MD: Lippincott Williams & Wilkins. pp. 590–1. ISBN 0-7817-6879-9.
{{#subobject:
|Page Name=Tranylcypromine |Pill Name=No _image.jpg |Drug Name=Tranylcypromine |Pill Ingred=cellulose, microcrystalline, anhydrous citric acid, croscarmellose sodium, d&c red no. 7, fd&c blue no. 2, fd&c red no. 40, fd&c yellow no. 6, gelatin, lactose, magnesium stearate, talc, titanium dioxide|+sep=; |Pill Imprint=PARNATE;SB |Pill Dosage=10.00 mg |Pill Color=Red|+sep=; |Pill Shape=Round |Pill Size (mm)=4.00 |Pill Scoring=1 |Pill Image= |Drug Author=Covis Pharmaceuticals Inc |NDC=24987-447-10
}}
{{#subobject:
|Label Page=Tranylcypromine |Label Name=Tranyl1.PNG
}}
{{#subobject:
|Label Page=Tranylcypromine |Label Name=Tranyl2.PNG
}}