Carbinoxamine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sree Teja Yelamanchili, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Carbinoxamine is a H1-blocking agent that is FDA approved for the treatment of seasonal and perennial allergic rhinitis, vasomotor rhinitis, allergic conjunctivitis due to inhalant allergens and foods, mild uncomplicated allergic skin manifestations of urticaria and angioedema, and dermatographism. Common adverse reactions include epigastric pain, coordination problem, dizziness, sedation, somnolence, and excessive bronchial secretions.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Seasonal and perennial allergic rhinitis.
- Vasomotor rhinitis.
- Allergic conjunctivitis due to inhalant allergens and foods.
- Mild, uncomplicated allergic skin manifestations of urticaria and angioedema.
- Dermatographism.
- As therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled.
- Amelioration of the severity of allergic reactions to blood or plasma.
Dosage
DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND THE RESPONSE OF THE PATIENT.
Carbinoxamine maleate dosage should be based on the severity of the condition and the response of the patient. The drug is well tolerated in adult doses as high as 24 mg daily, in divided doses, over prolonged periods. On the other hand, some patients respond to as little as 4 mg daily.
Clinical experience suggests the following dosage schedules:
Tablets
Usual Adult Dosage:
- 1 or 2 tablets (4 to 8 mg) 3 to 4 times daily.
Oral Solution
Usual Adult Dosage:
- 1 or 2 teaspoonfuls (4 to 8 mg) 3 to 4 times daily.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Carbinoxamine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Carbinoxamine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
- Seasonal and perennial allergic rhinitis.
- Vasomotor rhinitis.
- Allergic conjunctivitis due to inhalant allergens and foods.
- Mild, uncomplicated allergic skin manifestations of urticaria and angioedema.
- Dermatographism.
- As therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled.
- Amelioration of the severity of allergic reactions to blood or plasma.
Dosage
Tablets
Usual Child’s Dosage:
- Six to eleven years – 1/2 to 1 tablet (2 to 4 mg) 3 to 4 times daily.
Oral Solution
Usual Child’s Dosage:
- Approximately 0.2 to 0.4 mg/kg/day, divided into 3 to 4 doses
- Six to eleven years – 1/2 to 1 teaspoonful (2 to 4 mg) 3 to 4 times daily.
Dosing for children 2 to 5 years of age should be based on weight whenever possible. The usual dosage for children 2 to 5 years of age is approximately 0.2 to 0.4 mg/kg/day, divided into 3 to 4 daily doses. In general, this corresponds to a dose of 1/4 to 1/2 teaspoonful (1 to 2 mg) 3 to 4 times daily.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Carbinoxamine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Carbinoxamine in pediatric patients.
Contraindications
Carbinoxamine maleate is contraindicated in:
- Children younger than 2 years of age.
- Nursing mothers.
- Patients who are hypersensitive to the drug or on monoamine oxidase inhibitor therapy.
Warnings
Deaths have been reported in children less than 2 years of age who were taking antihistamines, including carbinoxamine-containing drug products, therefore, carbinoxamine maleate is contraindicated in children younger than 2 years of age.
Antihistamines should be used with considerable caution in patients with: narrow angle glaucoma, stenosing peptic ulcer, symptomatic prostatic hypertrophy, bladder neck obstruction, pyloroduodenal obstruction.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Carbinoxamine Clinical Trials Experience in the drug label.
Postmarketing Experience
Body as a Whole: Urticaria, drug rash, anaphylactic shock, photosensitivity, excessive perspiration, chills, dryness of mouth, nose and throat.
Cardiovascular: Hypotension, headache, palpitations, tachycardia, extrasystoles.
Hematologic: Hemolytic anemia, thrombocytopenia, agranulocytosis.
Central Nervous System: Sedation, sleepiness, dizziness, disturbed coordination, fatigue, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, euphoria, paresthesia, blurred vision, diplopia, vertigo, tinnitus, acute labyrinthitis, hysteria, neuritis, convulsions.
Gastrointestinal: Epigastric distress, anorexia, nausea, vomiting, diarrhea, constipation.
Urogenital: Urinary frequency, difficult urination, urinary retention, early menses.
Respiratory: Thickening of bronchial secretions, tightness of chest and wheezing, nasal stuffiness.
Drug Interactions
Monoamine oxidase inhibitors prolong and intensify the anticholinergic (drying) effects of antihistamines.
Carbinoxamine maleate has additive effects with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, etc.).
Interactions of carbinoxamine maleate with other drugs and the possibility of cardiac conduction effects on the QT interval have not been studied.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Animal reproductive studies have not been conducted with carbinoxamine maleate. It is also not known whether carbinoxamine maleate can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity.
Carbinoxamine maleate should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Carbinoxamine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Carbinoxamine during labor and delivery.
Nursing Mothers
Because of the higher risk of antihistamines for infants generally and for newborns and prematures in particular, use of carbinoxamine maleate is contraindicated in nursing mothers.
Pediatric Use
Carbinoxamine maleate is contraindicated in children younger than 2 years of age.
Carbinoxamine maleate may diminish mental alertness in children. In the young child, particularly, they may produce excitation.
Geriatic Use
Carbinoxamine maleate is more likely to cause dizziness, sedation, and hypotension in elderly patients (approximately 60 years or older). Sedating drugs may also cause confusion and over sedation in the elderly. Therefore, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Carbinoxamine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Carbinoxamine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Carbinoxamine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Carbinoxamine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Carbinoxamine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Carbinoxamine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
Vital signs (including respiration, pulse, blood pressure, and temperature) and EKG should be monitored in carbinoxamine overdosage.
IV Compatibility
There is limited information regarding the compatibility of Carbinoxamine and IV administrations.
Overdosage
Manifestations
Antihistamine overdosage reactions may vary from central nervous system depression to stimulation. Stimulation is particularly likely in children. Atropine-like signs and symptoms – dry mouth; fixed, dilated pupils; flushing; and gastrointestinal symptoms may also occur. Especially in infants and children, antihistamine overdosage may cause hallucinations, convulsions, or death.
The oral LD50 of carbinoxamine maleate in guinea pigs is 411 mg/kg.
Treatment
The treatment of overdosage with carbinoxamine maleate is essentially symptomatic and supportive. Vital signs (including respiration, pulse, blood pressure, and temperature) and EKG should be monitored. Induction of vomiting is not recommended. Activated charcoal should be given and gastric lavage should be considered after ingestion of a potentially life-threatening amount of drug.
In the presence of severe anticholinergic effects, physostigmine may be useful. Vasopressors may be used to treat hypotension.
Pharmacology
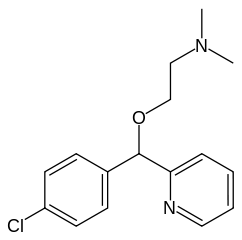
| |
Carbinoxamine
| |
| Systematic (IUPAC) name | |
| 2-[(4-Chlorophenyl)-pyridin-2-yl-methoxy]-N,N- dimethyl-ethanamine | |
| Identifiers | |
| CAS number | |
| ATC code | R06 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 290.788 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 10 to 20 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C |
| Legal status |
4 mg is FDA approved |
| Routes | Oral: 4 mg tablet or 4 mg/5 mL liquid |
Mechanism of Action
Carbinoxamine maleate is an antihistamine with anticholinergic (drying) and sedative properties. Antihistamines appear to compete with histamine for receptor sites on effector cells.
Structure
Carbinoxamine maleate is a histamine-H1 receptor blocking agent.
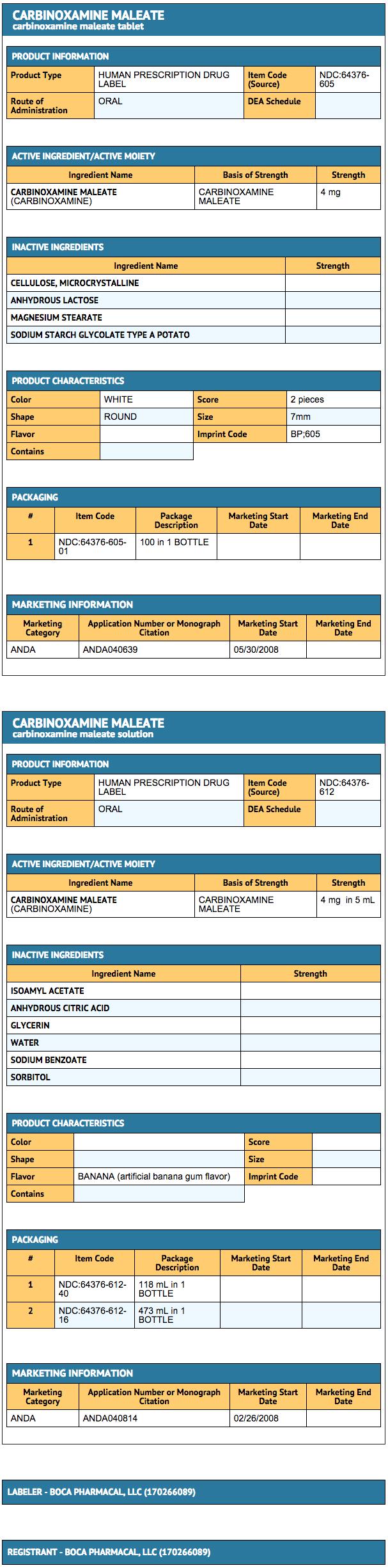
Each tablet contains 4 mg carbinoxamine maleate.
Inactive ingredients: anhydrous lactose, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate.
Each 5 mL (teaspoonful) of oral solution contains 4 mg carbinoxamine maleate.
Inactive ingredients: artificial banana bubble gum flavor, citric acid (anhydrous), glycerin, purified water, sodium benzoate and sorbitol solution.
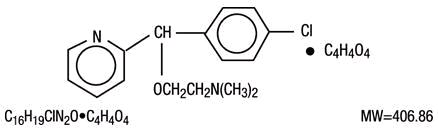
Carbinoxamine maleate is freely soluble in water.
Its structure is:
Pharmacodynamics
There is limited information regarding Carbinoxamine Pharmacodynamics in the drug label.
Pharmacokinetics
The pharmacological effects of carbinoxamine maleate after oral absorption have been shown to last approximately 4 hours.
Nonclinical Toxicology
There is limited information regarding Carbinoxamine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Carbinoxamine Clinical Studies in the drug label.
How Supplied
Carbinoxamine Maleate Tablets USP, 4 mg are supplied as white, round tablets scored and debossed “B” bisected “P” on one side and “605” on the other side, and supplied in bottles of 100 tablets, NDC 64376-605-01.
Carbinoxamine Maleate Oral Solution, 4 mg/5 mL is supplied as clear, colorless liquid with a banana bubble gum flavor, and is supplied in 4 oz bottles NDC 64376-612-40 and 16 oz bottles NDC 64376-612-16.
Manufactured for:
Boca Pharmacal, LLC
Coral Springs, FL 33065
www.bocapharmacal.com
1-800-354-8460
Rev. 08/13
Storage
Store at 20°-25°C (68°-77°F). [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container with a child-resistant closure as defined in the official compendium.
Images
Drug Images
{{#ask: Page Name::Carbinoxamine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Carbinoxamine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Carbinoxamine Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol may increase the drowsiness effect.
- Avoid alcoholic beverages while taking this product.
- Carbinoxamine maleate has additive effects with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, etc.).
Brand Names
- Histex PD
- Histex I/E
- Histex CT
- Pediox
- Carbihist
- Histuss PD
- Carboxine
- Mintex PD
Look-Alike Drug Names
There is limited information regarding Carbinoxamine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Carbinoxamine |Pill Name=Arbinoxa_NDC_637170870.jpg |Drug Name=Arbinoxa |Pill Ingred=CARBINOXAMINE MALEATE[CARBINOXAMINE]|+sep=; |Pill Imprint=CYP;870 |Pill Dosage=4 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=7 |Pill Scoring=2 |Pill Image= |Drug Author=Hawthorn Pharmaceuticals, Inc. |NDC=637170870
}}
{{#subobject:
|Page Name=Carbinoxamine |Pill Name=Carbinoxamine_Maleate_NDC_643760605.jpg |Drug Name=Carbinoxamine Maleate |Pill Ingred=CARBINOXAMINE MALEATE[CARBINOXAMINE]|+sep=; |Pill Imprint=BP;605 |Pill Dosage=4 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=7 |Pill Scoring=2 |Pill Image= |Drug Author=Boca Pharmacal, LLC |NDC=643760605
}}