Ormeloxifene
 | |
| Clinical data | |
|---|---|
| Trade names | Centron, Novex-DS, Saheli, Sevista |
| Synonyms | Centchroman |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 7 days |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
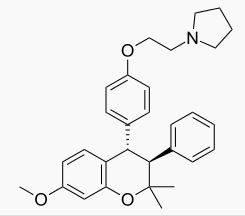
| Formula | C30H35NO3 |
| Molar mass | 457.604 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Template:Infobox Birth control
|
WikiDoc Resources for Ormeloxifene |
|
Articles |
|---|
|
Most recent articles on Ormeloxifene Most cited articles on Ormeloxifene |
|
Media |
|
Powerpoint slides on Ormeloxifene |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Ormeloxifene at Clinical Trials.gov Clinical Trials on Ormeloxifene at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Ormeloxifene
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Ormeloxifene Discussion groups on Ormeloxifene Patient Handouts on Ormeloxifene Directions to Hospitals Treating Ormeloxifene Risk calculators and risk factors for Ormeloxifene
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Ormeloxifene |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Ormeloxifene (also known as centchroman) is one of the selective estrogen receptor modulators,[1] or SERMs, a class of medication which acts on the estrogen receptor. It is best known as a non-hormonal, non-steroidal oral contraceptive which is taken once per week. In India, ormeloxifene has been available as birth control since the early 1990s, and it is currently marketed there under the trade name Saheli.[2] Ormeloxifene has also been licensed under the trade names Novex-DS, Centron and Sevista.
Medical uses
Ormeloxifene is primarily used as a contraceptive but may also be effective for dysfunctional uterine bleeding and advanced breast cancer.[3]
Birth control
Ormeloxifene may be used as a weekly oral contraceptive.[3] The weekly schedule is an advantage for women who prefer an oral contraceptive, but find it difficult or impractical to adhere to a daily schedule required by other oral contraceptives.
For the first twelve weeks of use, it is advised to take the ormeloxifene pill twice per week.[3] From the thirteenth week on, it is taken once per week.[3][4] The consensus is that backup protection in the first month is a cautious but sensible choice. A standard dose is 30 mg weekly, but 60 mg loading doses can reduce pregnancy rates by 38%.[5]
It has a failure rate of about 1-2% with ideal use which is slightly less effective than found for combined oral contraceptive pills.[6]
Other indications
- Ormeloxifene has also been tested in experimental setting as a treatment for menorrhagia.[7]
- use in treatment of mastalgia and fibroadenoma has also been described.[8]
Adverse effects
There are concerns that ormeloxifene may cause delayed mensturation.[9]
Method of action
Ormeloxifene is a SERM, or selective estrogen receptor modulator. In some parts of the body, its action is estrogenic (e.g., bones), in other parts of the body, its action is anti-estrogenic (e.g., uterus, breasts.[10][11]) It causes an asynchrony in the menstrual cycle between ovulation and the development of the uterine lining, although its exact mode of action is not well defined. In clinical trials, it caused ovulation to occur later than it normally would in some women,[6] but did not affect ovulation in the majority of women, while causing the lining of the uterus to build more slowly. It speeds the transport of any fertilized egg through the fallopian tubes more quickly than is normal.[6] Presumably, this combination of effects creates an environment such that if fertilization occurs, implantation will not be possible.[6]
Marketing
Ormeloxifene is only legally available in India as of 2009.[12]
Ormeloxifene has been tested and licensed as a form of birth control, as well as a treatment for dysfunctional uterine bleeding. It was first manufactured by Torrent Pharmaceuticals, and marketed as birth control under the trade name Centron. Centron was discontinued. A new license for ormeloxifene was issued to Hindustan Latex Ltd., which now manufactures ormeloxifene as birth control under the trade name Saheli, Novex and Novex-DS. Torrent Pharmaceuticals has resumed manufacture of ormeloxifene under the trade name Sevista, as a treatment for dysfunctional uterine bleeding.
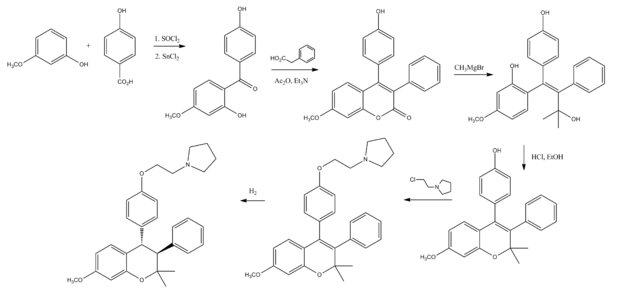
Synthesis
See also
- Hormonal contraception
- Levormeloxifene, a related SERM
References
- ↑ Makker, Annu; Tandon, Indu; Goel, Madhu Mati; Singh, Mastan; Singh, Man Mohan (2009). "Effect of ormeloxifene, a selective estrogen receptor modulator, on biomarkers of endometrial receptivity and pinopode development and its relation to fertility and infertility in Indian subjects". Fertility and Sterility. 91 (6): 2298–307. doi:10.1016/j.fertnstert.2008.04.018. PMID 18675966.
- ↑ "HLL - Product Overview".
- ↑ 3.0 3.1 3.2 3.3 Lal, J (April 2010). "Clinical pharmacokinetics and interaction of centchroman--a mini review". Contraception. 81 (4): 275–80. doi:10.1016/j.contraception.2009.11.007. PMID 20227542.
- ↑ http://www.reproline.jhu.edu/english/1fp/1advances/old/1centch/ceorvw.htm
- ↑ Lal J, Nitynand S, Asthana OP, Nagaraja NV, Gupta RC (January 2001). "Optimization of contraceptive dosage regimen of Centchroman". Contraception. 63 (1): 47–51. doi:10.1016/S0010-7824(00)00189-X. PMID 11257249.
- ↑ 6.0 6.1 6.2 6.3 Singh, M.M. (2001). "Centchroman, a selective estrogen receptor modulator, as a contraceptive and for the management of hormone-related clinical disorders". Medicinal Research Reviews. 21 (4): 302–47. doi:10.1002/med.1011. PMID 11410933.
- ↑ Kriplani A, Kulshrestha V, Agarwal N (August 2009). "Efficacy and safety of ormeloxifene in management of menorrhagia: a pilot study". J. Obstet. Gynaecol. Res. 35 (4): 746–52. doi:10.1111/j.1447-0756.2008.00987.x. PMID 19751337.
- ↑ Dhar A, Srivastava A (June 2007). "Role of centchroman in regression of mastalgia and fibroadenoma". World J Surg. 31 (6): 1178–84. doi:10.1007/s00268-007-9040-4. PMID 17431715.
- ↑ Shelly, W (March 2008). "Selective estrogen receptor modulators: an update on recent clinical findings". Obstetrical & gynecological survey. 63 (3): 163–81. doi:10.1097/OGX.0b013e31816400d7. PMID 18279543. Unknown parameter
|coauthors=ignored (help) - ↑ Gara Rishi Kumar, Konwar Rituraj, Bid Hemant K and MM Singh. In-vitro anti-cancer breast activity of ormeloxifene is mediated via induction of apoptosis and autophagy. 37th annual conference of the endocrine society of India. 30 nov-2 dec, 2007. Abstract p35.
- ↑ Nigam, Manisha; Ranjan, Vishal; Srivastava, Swasti; Sharma, Ramesh; Balapure, Anil K. (2008). "Centchroman induces G0/G1 arrest and Caspase-dependent Apoptosis involving Mitochondrial Membrane Depolarization in MCF-7 and MDA MB-231 Human Breast Cancer Cells". Life Sciences. 82 (11–12): 577–90. doi:10.1016/j.lfs.2007.11.028. PMID 18279897.
- ↑ Patil, Robin D. Tribhuwan & Benazir D. (2009). Body image : human reproduction and birth control : a tribal perspective. New Delhi: Discovery Pub. House. p. 20. ISBN 978-81-8356-388-8.
Further reading
- Ray, Suprabhat; Grover, Payara K.; Kamboj, Ved P.; Setty, B. S.; Kar, Amiya B.; Anand, Nitya (1976). "Antifertility agents. 12. Structure-activity relation of 3,4-diphenylchromenes and -chromans". Journal of Medicinal Chemistry. 19 (2): 276–9. doi:10.1021/jm00224a014. PMID 1249807.
External links
- United States National Library of Medicine Centchroman entry in the Medical Subject Headings (MeSH) database
- Reproductive Health Online, a Johns Hopkins University affiliate providing information on Centchroman
- Saheli manufacturer's website - Product details
- Central Drug Research Institute, Lucknow, India: a government-funded laboratory, conducting R&D on Centchroman as birth control.
- Ministry of Health and Family Welfare - Indian government site; information about availability of Saheli.
Template:Estrogens
Template:Birth control methods
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Pages with citations using unsupported parameters
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Use dmy dates from September 2011
- Articles with invalid date parameter in template
- Drug
- Hormonal contraception
- Selective estrogen receptor modulators
- Drugs acting on the genito-urinary system
- Pyrrolidines
- Phenol ethers
- Chromanes