Baricitinib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Zach Leibowitz [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
SERIOUS INFECTIONS, MALIGNANCY, AND THROMBOSIS
See full prescribing information for complete Boxed Warning.
SERIOUS INFECTIONS
MALIGNANCIES
THROMBOSIS
|
Overview
Baricitinib is a Janus kinase (JAK) inhibitor that is FDA approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more TNF antagonist therapies. There is a Black Box Warning for this drug as shown here. Common adverse reactions include upper respiratory tract infections, nausea, herpes simplex, and herpes zoster.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Baricitinib is indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF) antagonist therapies.
- Limitation of Use: Use of baricitinib in combination with other JAK inhibitors, biologic disease-modifying antirheumatic drugs (DMARDs), or with potent immunosuppressants such as azathioprine and cyclosporine is not recommended.
Dosage
- The recommended dose of baricitinib is 2 mg once daily.
- Baricitinib may be used as monotherapy or in combination with methotrexate or other DMARDs.
- Baricitinib is given orally with or without food.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding baricitinib Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding baricitinib Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The safety and effectiveness of baricitinib in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding baricitinib Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding baricitinib Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
None.
Warnings
|
SERIOUS INFECTIONS, MALIGNANCY, AND THROMBOSIS
See full prescribing information for complete Boxed Warning.
SERIOUS INFECTIONS
MALIGNANCIES
THROMBOSIS
|
Serious Infections
- Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other opportunistic pathogens have been reported in rheumatoid arthritis patients receiving baricitinib. The most common serious infections reported with baricitinib included pneumonia, herpes zoster, and urinary tract infection. Among opportunistic infections, tuberculosis, multidermatomal herpes zoster, esophageal candidiasis, pneumocystosis, acute histoplasmosis, cryptococcosis, cytomegalovirus, and BK virus were reported with baricitinib. Some patients have presented with disseminated rather than localized disease, and were often taking concomitant immunosuppressants such as methotrexate or corticosteroids.
- Avoid use of baricitinib in patients with an active, serious infection, including localized infections. Consider the risks and benefits of treatment prior to initiating baricitinib in patients:
- with chronic or recurrent infection
- who have been exposed to tuberculosis
- with a history of a serious or an opportunistic infection
- who have resided or traveled in areas of endemic tuberculosis or endemic mycoses; or
- with underlying conditions that may predispose them to infection.
- Closely monitor patients for the development of signs and symptoms of infection during and after treatment with baricitinib. Interrupt baricitinib if a patient develops a serious infection, an opportunistic infection, or sepsis. A patient who develops a new infection during treatment with baricitinib should undergo prompt and complete diagnostic testing appropriate for an immunocompromised patient; appropriate antimicrobial therapy should be initiated, the patient should be closely monitored, and baricitinib should be interrupted if the patient is not responding to therapy. Do not resume baricitinib until the infection is controlled.
Tuberculosis
- Evaluate and test patients for latent or active infection prior to administration of baricitinib. Patients with latent tuberculosis (TB) should be treated with standard antimycobacterial therapy before initiating baricitinib.
- Baricitinib should not be given to patients with active TB. Consider anti-TB therapy prior to initiation of baricitinib in patients with a history of latent or active TB in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent TB but who have risk factors for TB infection. Consultation with a physician with expertise in the treatment of TB is recommended to aid in the decision about whether initiating anti-TB therapy is appropriate for an individual patient.
- Monitor patients for the development of signs and symptoms of TB, including patients who tested negative for latent TB infection prior to initiating therapy.
Viral Reactivation
- Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), were reported in clinical studies with baricitinib. If a patient develops herpes zoster, interrupt baricitinib treatment until the episode resolves.
- The impact of baricitinib on chronic viral hepatitis reactivation is unknown. Patients with evidence of active hepatitis B or C infection were excluded from clinical trials. Patients who were positive for hepatitis C antibody but negative for hepatitis C virus RNA were permitted to enroll. Patients with positive hepatitis B surface antibody and hepatitis B core antibody, without hepatitis B surface antigen, were permitted to enroll; such patients should be monitored for expression of hepatitis B virus (HBV) DNA. Should HBV DNA be detected, consult with a hepatologist. Perform screening for viral hepatitis in accordance with clinical guidelines before starting therapy with baricitinib.
Malignancy and Lymphoproliferative Disorders
- Consider the risks and benefits of baricitinib treatment prior to initiating therapy in patients with a known malignancy other than a successfully treated non-melanoma skin cancer (NMSC) or when considering continuing baricitinib in patients who develop a malignancy. Malignancies were observed in clinical studies of baricitinib.
Non-melanoma skin cancers
- Non-melanoma skin cancers (NMSCs) have been reported in patients treated with baricitinib. Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
Thrombosis
- Thrombosis, including deep venous thrombosis (DVT) and pulmonary embolism (PE), has been observed at an increased incidence in patients treated with baricitinib compared to placebo. In addition, arterial thrombosis events in the extremities have been reported in clinical studies with baricitinib. Many of these adverse events were serious and some resulted in death. There was no clear relationship between platelet count elevations and thrombotic events. Baricitinib should be used with caution in patients who may be at increased risk of thrombosis. If clinical features of DVT/PE or arterial thrombosis occur, patients should be evaluated promptly and treated appropriately.
Gastrointestinal Perforations
- Events of gastrointestinal perforation have been reported in clinical studies with baricitinib, although the role of JAK inhibition in these events is not known.
- Baricitinib should be used with caution in patients who may be at increased risk for gastrointestinal perforation (e.g., patients with a history of diverticulitis). Patients presenting with new onset abdominal symptoms should be evaluated promptly for early identification of gastrointestinal perforation.
Laboratory Abnormalities
- Neutropenia – Treatment with baricitinib was associated with an increased incidence of neutropenia (ANC less than 1000 cells/mm3) compared to placebo. Avoid initiation or interrupt baricitinib treatment in patients with an ANC less than 1000 cells/mm3. Evaluate at baseline and thereafter according to routine patient management. For recommended modifications based on ANC.
- Lymphopenia – ALC less than 500 cells/mm3 were reported in baricitinib clinical trials. Lymphocyte counts less than the lower limit of normal were associated with infection in patients treated with baricitinib, but not placebo.
- Avoid initiation or interrupt baricitinib treatment in patients with an ALC less than 500 cells/mm3. Evaluate at baseline and thereafter according to routine patient management. For recommended modifications based on ALC results.
- Anemia – Decreases in hemoglobin levels to less than 8 g/dL were reported in baricitinib clinical trials. Avoid initiation or interrupt baricitinib treatment in patients with hemoglobin less than 8 g/dL. Evaluate at baseline and thereafter according to routine patient management. For recommended modifications based on hemoglobin results.
- Liver Enzyme Elevations – Treatment with baricitinib was associated with increased incidence of liver enzyme elevation compared to placebo. Increases to greater than or equal to 5x and greater than or equal to 10x upper limit of normal (ULN) were observed for both ALT and AST in patients in baricitinib clinical trials.
- Evaluate at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. If increases in ALT or AST are observed and drug-induced liver injury is suspected, interrupt baricitinib until this diagnosis is excluded.
- Lipid Elevations – Treatment with baricitinib was associated with increases in lipid parameters, including total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol. Assessment of lipid parameters should be performed approximately 12 weeks following baricitinib initiation.
- Manage patients according to clinical guidelines for the management of hyperlipidemia.
Vaccinations
- Avoid use of live vaccines with baricitinib.
- Update immunizations in agreement with current immunization guidelines prior to initiating baricitinib therapy.
Adverse Reactions
Clinical Trials Experience
- Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not predict the rates observed in a broader patient population in clinical practice.
- The following data include six randomized double-blind placebo-controlled studies (three Phase 2, three Phase 3) and a long-term extension study. All patients had moderately to severely active RA. Patients were randomized to placebo (1070 patients), baricitinib 2 mg (479 patients), or baricitinib 4 mg (997 patients).
- Patients could be switched to baricitinib 4 mg from placebo or baricitinib 2 mg from as early as Week 12 depending on the study design. All patients initially randomized to placebo were switched to baricitinib 4 mg by Week 24.
- During the 16-week treatment period, adverse events leading to discontinuation of treatment were reported by 35 patients (11.4 events per 100 patient-years) treated with placebo, 17 patients (12.1 events per 100 patient-years) with baricitinib 2 mg, and 40 patients (13.4 events per 100 patient-years) treated with baricitinib 4 mg.
- During 0 to 52 week exposure, adverse events leading to discontinuation of treatment were reported by 31 patients (9.2 events per 100 patient-years) with baricitinib 2 mg, and 92 patients (10.2 events per 100 patient-years) treated with baricitinib 4 mg.
- Overall Infections – During the 16-week treatment period, infections were reported by 253 patients (82.1 events per 100 patient-years) treated with placebo, 139 patients (99.1 events per 100 patient-years) treated with baricitinib 2 mg, and 298 patients (100.1 events per 100 patient-years) treated with baricitinib 4 mg.
- During 0 to 52 week exposure, infections were reported by 200 patients (59.6 events per 100 patients-years) treated with baricitinib 2 mg, and 500 patients (55.3 events per 100 patient-years) treated with baricitinib 4 mg.
- In the 0 to 52 week exposure population, the most commonly reported infections with baricitinib were viral upper respiratory tract infection, upper respiratory tract infection, urinary tract infection, and bronchitis.
- Serious Infections – During the 16-week treatment period, serious infections were reported in 13 patients (4.2 events per 100 patient-years) treated with placebo, 5 patients (3.6 events per 100 patient-years) treated with baricitinib 2 mg, and 11 patients (3.7 events per 100 patient-years) treated with baricitinib 4 mg.
- During 0 to 52 week exposure, serious infections were reported in 14 patients (4.2 events per 100 patient-years) treated with baricitinib 2 mg and 32 patients (3.5 events per 100 patient-years) treated with baricitinib 4 mg.
- In the 0 to 52 week exposure population, the most commonly reported serious infections with baricitinib were pneumonia, herpes zoster, and urinary tract infection.
- Tuberculosis – During the 16-week treatment period, no events of tuberculosis were reported.
- During 0 to 52 week exposure, events of tuberculosis were reported in 0 patients treated with baricitinib 2 mg and 1 patient (0.1 per 100 patient-years) treated with baricitinib 4 mg.
- Cases of disseminated tuberculosis were also reported.
- Opportunistic Infections (excluding tuberculosis) – During the 16-week treatment period, opportunistic infections were reported in 2 patients (0.6 per 100 patient-years) treated with placebo, 0 patients treated with baricitinib 2 mg and 2 patients (0.7 per 100 patient-years) treated with baricitinib 4 mg.
- During 0 to 52 week exposure, opportunistic infections were reported in 1 patient (0.3 per 100 patient-years) treated with baricitinib 2 mg and 5 patients (0.6 per 100 patient-years) treated with baricitinib 4 mg.
- Malignancy – During the 16-week treatment period, malignancies excluding non-melanoma skin cancers (NMSC) were reported in 0 patients treated with placebo, 1 patient (0.7 per 100 patient-years) treated with baricitinib 2 mg, and 1 patient (0.3 per 100 patient-years) treated with baricitinib 4 mg.
- During the 0 to 52 week treatment period, malignancies excluding NMSC were reported in 2 patients (0.6 per 100 patient-years) treated with baricitinib 2 mg and 6 patients (0.7 per 100 patient-years) treated with baricitinib 4 mg.
- Venous Thrombosis – During the 16-week treatment period, venous thromboses (deep vein thrombosis or pulmonary embolism) were reported in 0 patients treated with placebo, 0 patients treated with baricitinib 2 mg, and 5 patients (1.7 per 100 patient-years) treated with baricitinib 4 mg.
- During the 0 to 52 week treatment period, venous thromboses were reported in 2 patients (0.6 per 100 patient-years) treated with baricitinib 2 mg and 7 patients (0.8 per 100 patient-years) treated with baricitinib 4 mg.
- Arterial Thrombosis – During the 16-week treatment period, arterial thromboses were reported in 1 patient treated with placebo (0.3 per 100 patient-years), 2 patients (1.4 per 100 patient-years) treated with baricitinib 2 mg, and 2 patients (0.7 per 100 patient-years) treated with baricitinib 4 mg.
- During the 0 to 52 week treatment period, arterial thromboses were reported in 3 patients (0.9 per 100 patient-years) treated with baricitinib 2 mg and 3 patients (0.3 per 100 patient-years) treated with baricitinib 4 mg.
Laboratory Abnormalities
- Neutropenia – During the 16-week treatment period, neutrophil counts below 1000 cells/mm3 occurred in 0% of patients treated with placebo, 0.6% of patients treated with baricitinib 2 mg, and 0.3% of patients treated with baricitinib 4 mg. There were no neutrophil counts below 500 cells/mm3 observed in any treatment group.
- Platelet Elevations – During the 16-week treatment period, increases in platelet counts above 600,000 cells/mm3 occurred in 1.1% of patients treated with placebo, 1.1% of patients treated with baricitinib 2 mg, and 2.0% of patients treated with baricitinib 4 mg. Mean platelet count increased by 3000 cells/mm3 at 16 weeks in patients treated with placebo, by 15,000 cells/mm3 at 16 weeks in patients treated with baricitinib 2 mg and by 23,000 cells/mm3 in patients treated with baricitinib 4 mg.
- Liver Enzyme Elevations – Events of increases in liver enzymes greater than or equal to 3x ULN were observed in patients treated with baricitinib.
- During the 16-week treatment period, ALT elevations greater than or equal to 3x ULN occurred in 1.0% of patients treated with placebo, 1.7% of patients treated with baricitinib 2 mg, and 1.4% of patients treated with baricitinib 4 mg.
- During the 16-week treatment period, AST elevations greater than or equal to 3x ULN occurred in 0.8% of patients treated with placebo, 1.3% of patients treated with baricitinib 2 mg, and 0.8% of patients treated with baricitinib 4 mg.
- In a phase 3 study of DMARD naive patients, during the 24-week treatment period, ALT and AST elevations ≥3x ULN occurred in 1.9% and 0% of patients treated with methotrexate monotherapy, 1.9% and 1.3% of patients treated with baricitinib 4 mg monotherapy, and 4.7% and 1.9% of patients treated with baricitinib 4 mg plus methotrexate.
- Lipid Elevations – In controlled clinical trials, baricitinib treatment was associated with dose-related increases in lipid parameters including total cholesterol, triglycerides, LDL cholesterol, and HDL cholesterol. Elevations were observed at 12 weeks and remained stable thereafter. During the 12-week treatment period, changes in lipid parameters are summarized below:
- Mean LDL cholesterol increased by 8 mg/dL in patients treated with baricitinib 2 mg and by 14 mg/dL in patients treated with baricitinib 4 mg.
- Mean HDL cholesterol increased by 7 mg/dL in patients treated with baricitinib 2 mg and by 9 mg/dL in patients treated with baricitinib 4 mg.
- The mean LDL/HDL ratio remained stable.
- Mean triglycerides increased by 7 mg/dL in patients treated with baricitinib 2 mg and by 15 mg/dL in patients treated with baricitinib 4 mg.
- Creatine Phosphokinase (CPK) – baricitinib treatment was associated with increases in CPK within one week of starting baricitinib and plateauing after 8 to 12 weeks. At 16 weeks, the mean change in CPK for baricitinib 2 mg and baricitinib 4 mg was 37 IU/L and 52 IU/L, respectively.
- Creatinine – In controlled clinical trials, dose-related increases in serum creatinine were observed with baricitinib treatment. At 52 weeks, the mean increase in serum creatinine was less than 0.1 mg/dL with baricitinib 4 mg. The clinical significance of the observed serum creatinine increases is unknown.
Other Adverse Reactions
- Other adverse reactions are summarized in TABLE 4.
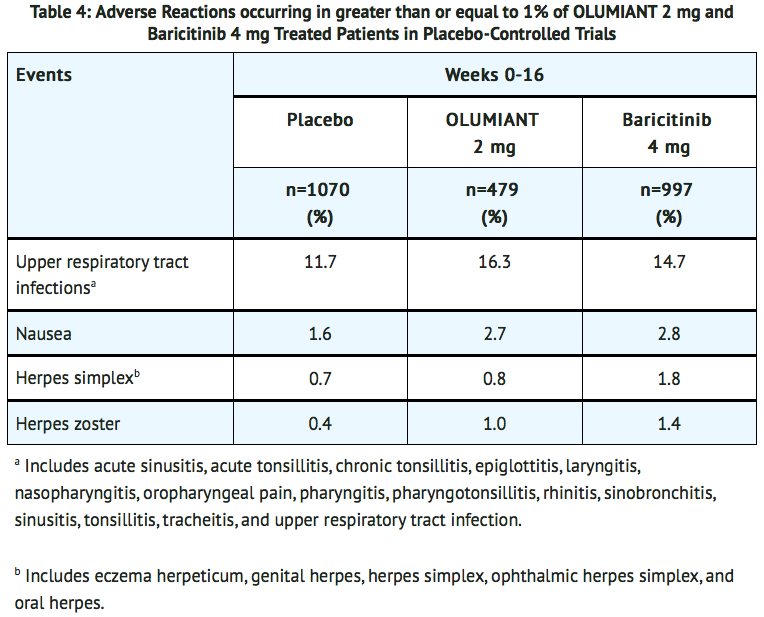
- Additional adverse drug reactions occurring in fewer than 1% of patients: acne.
Postmarketing Experience
There is limited information regarding Baricitinib Postmarketing Experience in the drug label.
Drug Interactions
Strong OAT3 Inhibitors
- Baricitinib exposure is increased when baricitinib is co-administered with strong OAT3 inhibitors (such as probenecid).
Other JAK Inhibitors or Biologic DMARDs
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- The limited human data on use of baricitinib in pregnant women are not sufficient to inform a drug-associated risk for major birth defects or miscarriage. In animal embryo-fetal development studies, oral baricitinib administration to pregnant rats and rabbits at exposures equal to and greater than approximately 20 and 84 times the maximum recommended human dose (MRHD), respectively, resulted in reduced fetal body weights, increased embryolethality (rabbits only), and dose-related increases in skeletal malformations. No developmental toxicity was observed in pregnant rats and rabbits treated with oral baricitinib during organogenesis at approximately 5 and 13 times the exposure at the MRHD, respectively. In a pre- and post-natal development study in pregnant female rats, oral baricitinib administration at exposures approximately 43 times the MRHD resulted in reduction in pup viability (increased incidence of stillborn pups and early neonatal deaths), decreased fetal birth weight, reduced fetal body weight gain, decreased cytotoxic T cells on post-natal day (PND) 35 with evidence of recovery by PND 65, and developmental delays that might be attributable to decreased body weight gain. No developmental toxicity was observed at an exposure approximately 9 times the exposure at the MRHD.
- The estimated background risk of major birth defects and miscarriage for the indicated population(s) are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Animal Data
- In an embryofetal development study in pregnant rats, dosed orally during the period of organogenesis from gestation days 6 to 17, baricitinib was teratogenic (skeletal malformations that consisted of bent limb bones and rib anomalies) at exposures equal to or greater than approximately 20 times the MRHD (on an AUC basis at maternal oral doses of 10 mg/kg/day and higher). No developmental toxicity was observed in rats at an exposure approximately 5 times the MRHD (on an AUC basis at a maternal oral dose of 2 mg/kg/day).
- In an embryofetal development study in pregnant rabbits, dosed orally during the period of organogenesis from gestation days 7 to 20, embryolethality, decreased fetal body weights, and skeletal malformations (rib anomalies) were observed in the presence of maternal toxicity at an exposure approximately 84 times the MRHD (on an AUC basis at a maternal oral dose of 30 mg/kg/day). Embryolethality consisted of increased post-implantation loss that was due to elevated incidences of both early and late resorptions. No developmental toxicity was observed in rabbits at an exposure approximately 12 times the MRHD (on an AUC basis at a maternal oral dose of 10 mg/kg/day).
- In a pre- and post-natal development study in pregnant female rats dosed orally from gestation day 6 through lactation day 20, adverse findings observed in pups included decreased survival from birth to post-natal day 4 (due to increased stillbirths and early neonatal deaths), decreased birth weight, decreased body weight gain during the pre-weaning phase, increased incidence of malrotated forelimbs during the pre-weaning phase, and decreased cytotoxic T cells on PND 35 with recovery by PND 65 at exposures approximately 43 times the MRHD (on an AUC basis at a maternal oral dose of 25 mg/kg/day). Developmental delays (that may be secondary to decreased body weight gain) were observed in males and females at exposures approximately 43 times the MRHD (on an AUC basis at a maternal oral dose of 25 mg/kg/day). These findings included decreased forelimb and hindlimb grip strengths, and delayed mean age of sexual maturity. No developmental toxicity was observed in rats at an exposure approximately 9 times the MRHD (on an AUC basis at a maternal oral dose of 5 mg/kg/day).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Baricitinib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Baricitinib during labor and delivery.
Nursing Mothers
Risk Summary
- No information is available on the presence of baricitinib in human milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. Baricitinib is present in the milk of lactating rats. Due to species-specific differences in lactation physiology, the clinical relevance of these data are not clear. Because of the potential for serious adverse reactions in nursing infants, advise an baricitinib-treated woman not to breastfeed.
Data
- A single oral dose of 25 mg/kg radiolabeled baricitinib was administered to lactating female Sprague-Dawley rats on post-partum day 13. Drug exposure was approximately 45-fold greater in milk than in plasma based on AUC0-t values.
Pediatric Use
- The safety and effectiveness of baricitinib in pediatric patients have not been established.
Geriatic Use
- Of the 3100 patients treated in the four phase 3 studies, a total of 537 rheumatoid arthritis patients were 65 years of age and older, including 71 patients 75 years and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
- Baricitinib is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Baricitinib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Baricitinib with respect to specific racial populations.
Renal Impairment
- Renal function was found to significantly affect baricitinib exposure. Baricitinib is not recommended for use in patients with estimated GFR of less than 60 mL/min/1.73 m2
Hepatic Impairment
- No dose adjustment is necessary in patients with mild or moderate hepatic impairment. The use of baricitinib has not been studied in patients with severe hepatic impairment and is therefore not recommended.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Baricitinib in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Baricitinib in patients who are immunocompromised.
Administration and Monitoring
Administration
Dosage in Rheumatoid Arthritis
- The recommended dose of baricitinib is 2 mg once daily.
- Baricitinib may be used as monotherapy or in combination with methotrexate or other DMARDs.
- Baricitinib is given orally with or without food.
General Considerations for Administration
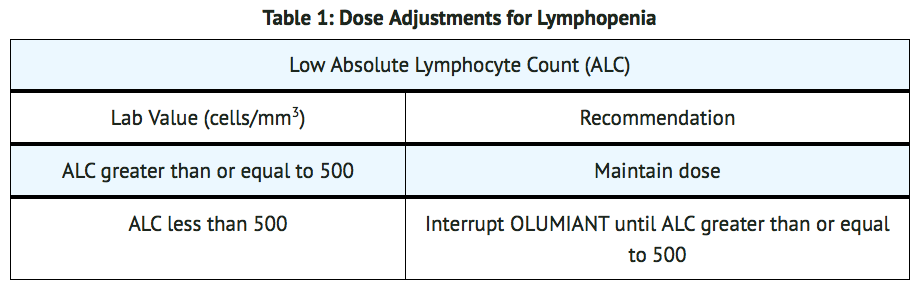
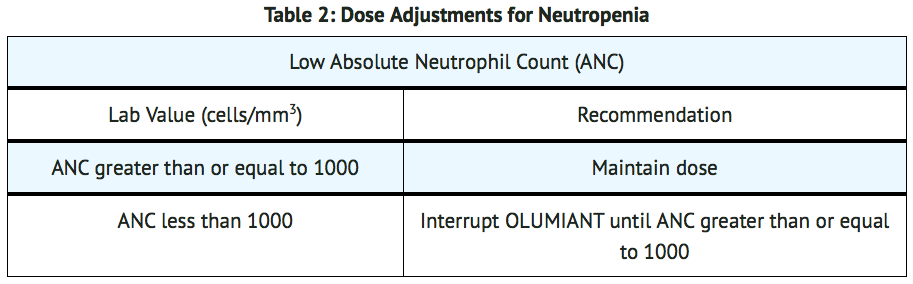
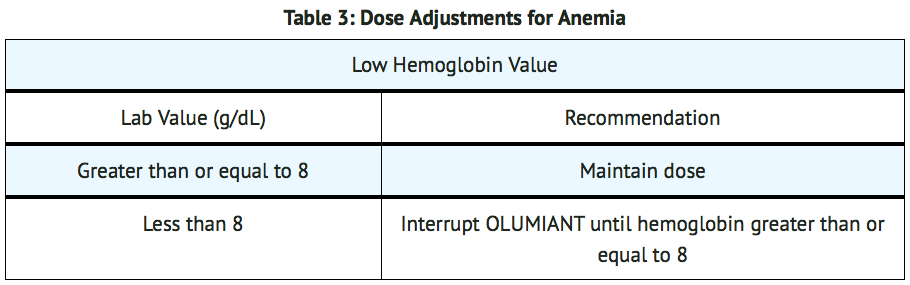
- Baricitinib initiation is not recommended in patients with an absolute lymphocyte count (ALC) less than 500 cells/mm3, absolute neutrophil count (ANC) less than 1000 cells/mm3, or hemoglobin level less than 8 g/dL.
- Avoid use of baricitinib in patients with active, serious infection, including localized infections.
- Prior to initiating baricitinib, test patients for latent tuberculosis (TB). If positive, consider treating for TB prior to baricitinib use.
Monitoring
Dose Modifications Due to Serious Infections and Cytopenias
- If a patient develops a serious infection, hold treatment with baricitinib until the infection is controlled.
- Modify dosage in cases of lymphopenia, neutropenia or anemia (TABLES 1, 2, and 3). For treatment initiation criteria.
Dose Modifications in Patients with Renal or Hepatic Impairment
- Baricitinib is not recommended for use in patients with estimated GFR of less than 60 mL/min/1.73 m2.
- Baricitinib is not recommended for use in patients with severe hepatic impairment.
Dose Modifications Due to Drug Interactions
- Baricitinib is not recommended for use in patients taking strong Organic Anion Transporter 3 (OAT3) inhibitors, such as probenecid.
IV Compatibility
There is limited information regarding the compatibility of Baricitinib and IV administrations.
Overdosage
- Single doses up to 40 mg and multiple doses of up to 20 mg daily for 10 days have been administered in clinical trials without dose-limiting toxicity. Pharmacokinetic data of a single dose of 40 mg in healthy volunteers indicate that more than 90% of the administered dose is expected to be eliminated within 24 hours.
- In case of an overdose, it is recommended that the patient should be monitored for signs and symptoms of adverse reactions. Patients who develop adverse reactions should receive appropriate treatment.
Pharmacology
| |
Baricitinib
| |
| Systematic (IUPAC) name | |
| 2-[1-Ethylsulfonyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]azetidin-3-yl]acetonitrile | |
| Identifiers | |
| CAS number | |
| ATC code | L04 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 371.42 g/mol |
| SMILES | & |
| Synonyms | INCB28050, LY3009104 |
| Pharmacokinetic data | |
| Bioavailability | 79% |
| Protein binding | 50% |
| Metabolism | CYP3A4 (<10%) |
| Half life | 12.5 hours |
| Excretion | 75% urine, 20% faeces |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
? |
| Legal status |
Template:Unicode Prescription only |
| Routes | By mouth (tablets) |
Mechanism of Action
- Baricitinib is a Janus kinase (JAK) inhibitor. JAKs are intracellular enzymes which transmit signals arising from cytokine or growth factor-receptor interactions on the cellular membrane to influence cellular processes of hematopoiesis and immune cell function. Within the signaling pathway, JAKs phosphorylate and activate Signal Transducers and Activators of Transcription (STATs) which modulate intracellular activity including gene expression. Baricitinib modulates the signaling pathway at the point of JAKs, preventing the phosphorylation and activation of STATs.
- JAK enzymes transmit cytokine signaling through their pairing (e.g., JAK1/JAK2, JAK1/JAK3, JAK1/TYK2, JAK2/JAK2, JAK2/TYK2). In cell-free isolated enzyme assays, baricitinib had greater inhibitory potency at JAK1, JAK2 and TYK2 relative to JAK3. In human leukocytes, baricitinib inhibited cytokine induced STAT phosphorylation mediated by JAK1/JAK2, JAK1/JAK3, JAK1/TYK2, or JAK2/TYK2 with comparable potencies. However, the relevance of inhibition of specific JAK enzymes to therapeutic effectiveness is not currently known.
Structure
- Baricitinib has an empirical formula of C16H17N7O2S and a molecular weight of 371.42. Baricitinib has the following structural formula:
Pharmacodynamics
- Baricitinib inhibition of IL-6 induced STAT3 phosphorylation – Baricitinib administration resulted in a dose dependent inhibition of IL-6 induced STAT3 phosphorylation in whole blood from healthy subjects with maximal inhibition observed approximately 1 hour after dosing, which returned to near baseline by 24 hours. Similar levels of inhibition were observed using either IL-6 or TPO as the stimulus.
- Immunoglobulins – Mean serum IgG, IgM, and IgA values decreased by 12 weeks after starting treatment with baricitinib, and remained stable through at least 52 weeks. For most patients, changes in immunoglobulins occurred within the normal reference range.
- C-reactive protein – In patients with rheumatoid arthritis, decreases in serum C-reactive protein (CRP) were observed as early as one week after starting treatment with baricitinib and were maintained throughout dosing.
- Cardiac Electrophysiology – At a dose 10 times the maximum recommended dose, baricitinib does not prolong the QT interval to any clinically relevant extent.
Pharmacokinetics
- Following oral administration of baricitinib, peak plasma concentrations are reached approximately at 1 hour. A dose-proportional increase in systemic exposure was observed in the therapeutic dose range. The pharmacokinetics of baricitinib do not change over time. Steady-state concentrations are achieved in 2 to 3 days with minimal accumulation after once-daily administration.
- Absorption – The absolute bioavailability of baricitinib is approximately 80%. An assessment of food effects in healthy subjects showed that a high-fat meal decreased the mean AUC and Cmax of baricitinib by approximately 11% and 18%, respectively, and delayed the tmax by 0.5 hours. Administration with meals is not associated with a clinically relevant effect on exposure. In clinical studies, baricitinib was administered without regard to meals.
- Distribution – After intravenous administration, the volume of distribution is 76 L, indicating distribution of baricitinib into tissues. Baricitinib is approximately 50% bound to plasma proteins and 45% bound to serum proteins. Baricitinib is a substrate of the Pgp, BCRP, OAT3 and MATE2-K transporters, which play roles in drug distribution.
- Elimination – The total body clearance of baricitinib is 8.9 L/h in patients with RA. Elimination half-life in patients with rheumatoid arthritis is approximately 12 hours.
- Metabolism – Approximately 6% of the orally administered baricitinib dose is identified as metabolites (three from urine and one from feces), with CYP3A4 identified as the main metabolizing enzyme. No metabolites of baricitinib were quantifiable in plasma.
- Excretion – Renal elimination is the principal clearance mechanism for baricitinib through filtration and active secretion as baricitinib is identified as a substrate of OAT3, Pgp, BCRP and MATE2-K from in vitro studies. In a clinical pharmacology study, approximately 75% of the administered dose was eliminated in the urine, while about 20% of the dose was eliminated in the feces. Baricitinib was excreted predominately as unchanged drug in urine (69%) and feces (15%).
Specific Populations:
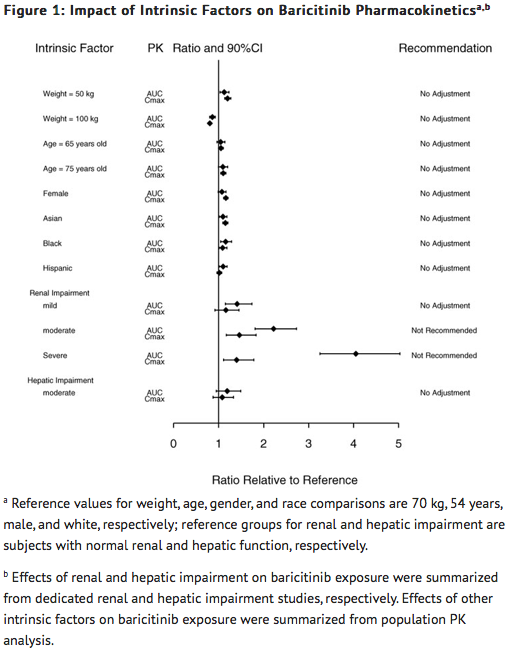
Effects of Body Weight, Gender, Race, and Age
- Body weight, gender, race, ethnicity, and age did not have a clinically relevant effect on the PK (AUC and Cmax) of baricitinib (FIGURE 1). The mean effects of intrinsic factors on PK parameters (AUC and Cmax) were generally within the inter-subject PK variability of baricitinib. The inter-subject variabilities (% coefficients of variation) in AUC and Cmax of baricitinib are approximately 41% and 22%, respectively.
Renal Impairment
- Baricitinib systemic exposure in AUC was increased by 1.41-, 2.22-, 4.05- and 2.41-fold for mild, moderate, severe, and ESRD (with hemodialysis) renal impairment sub-groups, respectively, compared to subjects with normal renal function. The corresponding values for increase in Cmax were 1.16-, 1.46-, 1.40- and 0.88-fold, respectively (FIGURE 1).
Hepatic Impairment
- Baricitinib systemic exposure and Cmax increased by 1.19- and 1.08-fold for the moderate hepatic impairment group, respectively, compared to subjects with normal hepatic function (FIGURE 1).
Drug Interactions:
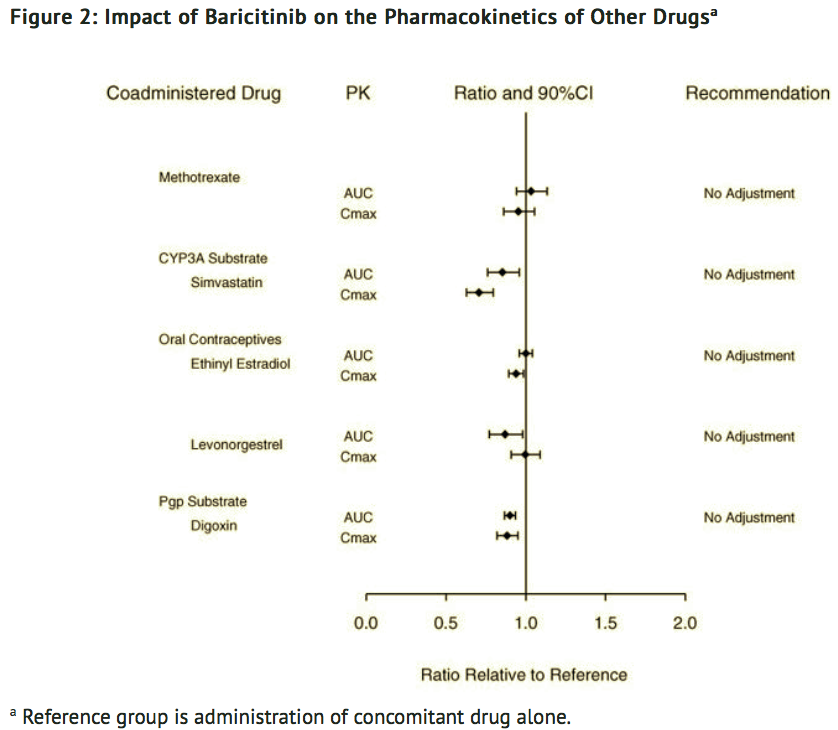
Potential for Baricitinib to Influence the PK of Other Drugs
- In vitro, baricitinib did not significantly inhibit or induce the activity of cytochrome P450 enzymes (CYPs 3A, 1A2, 2B6, 2C8, 2C9, 2C19, and 2D6). In clinical pharmacology studies, there were no clinically meaningful changes in the pharmacokinetics (PK) of simvastatin, ethinyl estradiol, or levonorgestrel (CYP3A substrates) when co-administered with baricitinib.
- In vitro studies suggest that baricitinib is not an inhibitor of the transporters, P-glycoprotein (Pgp) or Organic Anion Transporting Polypeptide (OATP) 1B1. In vitro data indicate baricitinib does inhibit organic anionic transporter (OAT) 1, OAT2, OAT3, organic cationic transporter (OCT) 1, OCT2, OATP1B3, Breast Cancer Resistance Protein (BCRP) and Multidrug and Toxic Extrusion Protein (MATE) 1 and MATE2-K, but clinically meaningful changes in the pharmacokinetics of drugs that are substrates for these transporters are unlikely. In clinical pharmacology studies there were no clinically meaningful effects on the PK of digoxin (Pgp substrate) or methotrexate (substrate of several transporters) when co-administered with baricitinib.
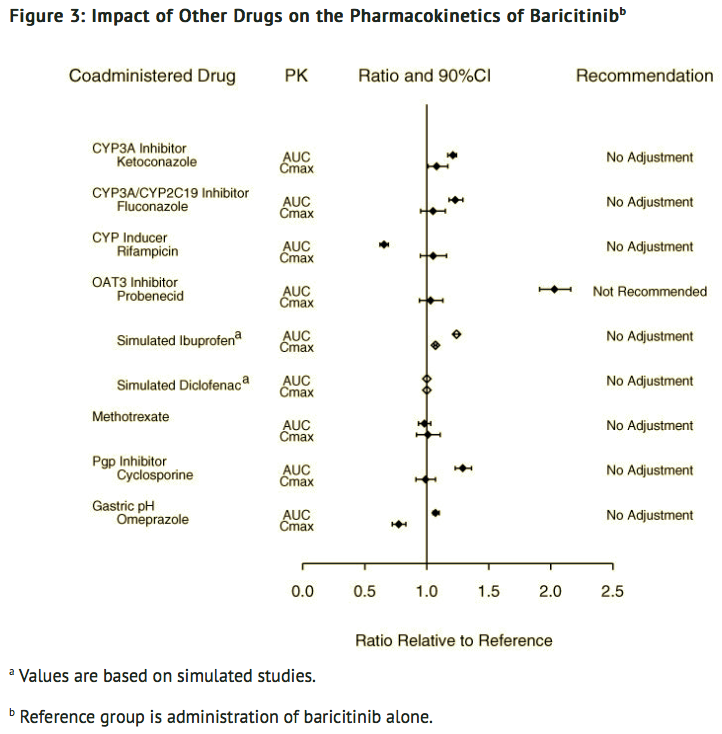
- Exposure changes of drugs following co-administration with baricitinib are shown in FIGURE 2.
Potential for Other Drugs to Influence the PK of Baricitinib
- In vitro studies suggest that baricitinib is a CYP3A4 substrate. In clinical pharmacology studies there was no effect on the PK of baricitinib when co-administered with ketoconazole (CYP3A inhibitor). There were no clinically meaningful changes in the PK of baricitinib when co-administered with fluconazole (CYP3A/CYP2C19/CYP2C9 inhibitor) or rifampicin (CYP3A inducer).
- In vitro studies suggest that baricitinib is a substrate for OAT3, Pgp, BCRP and MATE2-K. In a clinical study, probenecid administration (strong OAT3 inhibitor) resulted in an approximately 2-fold increase in baricitinib AUC0-∞ with no effect on Cmax and tmax. However, simulations with diclofenac and ibuprofen (OAT3 inhibitors with less inhibition potential) predicted minimal effect on the PK of baricitinib. In clinical pharmacology studies there was no clinically meaningful effect on the PK of baricitinib when co-administered with cyclosporine (Pgp and BCRP inhibitor). Co-administration with methotrexate (substrate of several transporters) did not have a clinically meaningful effect on the PK of baricitinib.
- Exposure changes of baricitinib following co-administration with CYP inhibitors or inducers, transporter inhibitors, as well as methotrexate and the proton pump inhibitor, omeprazole, are shown in FIGURE 3.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- The carcinogenic potential of baricitinib was evaluated in Sprague-Dawley rats and Tg.rasH2 mice. No evidence of tumorigenicity was observed in male or female rats that received baricitinib for 91 to 94 weeks at oral doses up to 8 or 25 mg/kg/day, respectively (approximately 12 and 55 times the MRHD on an AUC basis). No evidence of tumorigenicity was observed in Tg.rasH2 mice that received baricitinib for 26 weeks at oral doses up to 300 and 150 mg/kg/day in male and female mice, respectively.
- Baricitinib tested negative in the following genotoxicity assays: the in vitro bacterial mutagenicity assay (Ames assay), in vitro chromosome aberration assay in human peripheral blood lymphocytes, and in vivo rat bone marrow micronucleus assay.
- Fertility (achievement of pregnancy) was reduced in male and female rats that received baricitinib at oral doses of 50 and 100 mg/kg/day respectively (approximately 113 and 169 times the MRHD in males and females, respectively, on an AUC basis) based upon findings that 7 of 19 (36.8%) drug-treated females with evidence of mating were not gravid compared to 1 of 19 (5.3%) control females. It could not be determined from the study design if these findings were attributable to toxicities in one sex or both. Fertility was unaffected in male and female rats at oral doses of 15 mg/kg and 25 mg/kg, respectively (approximately 25 and 48 times the MRHD on an AUC basis). However, maintenance of pregnancy was adversely affected at these doses based upon findings of increased post-implantation losses (early resorptions) and decreased numbers of mean viable embryos per litter. The number of viable embryos was unaffected in female rats that received baricitinib at an oral dose of 5 mg/kg/day and were mated to males that received the same dose (approximately 8 times the MRHD on an AUC basis). Reproductive performance was unaffected in male and female rats that received baricitinib at oral doses up to 50 and 100 mg/kg/day respectively (approximately 113 and 169 times the MRHD in males and females, respectively, on an AUC basis).
Clinical Studies
- The baricitinib clinical development program included two dose-ranging trials and four confirmatory phase 3 trials. Although other doses have been studied, the recommended dose of baricitinib is 2 mg once daily.
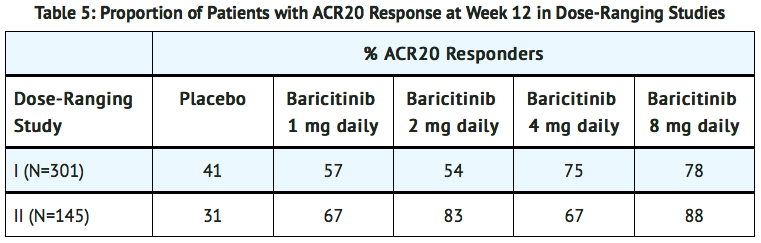
Dose-Ranging Studies
- The dose-ranging studies I (NCT01185353) and II (NCT01469013) included a 12-week randomized comparison of baricitinib 1, 2, 4, and 8 mg versus placebo in 301 and 145 patients, respectively.
- The results from the dose-ranging studies are shown in TABLE 5. In dose-ranging Study I, the observed ACR response was similar for baricitinib 1 and 2 mg daily and for baricitinib 4 and 8 mg daily, with the highest response for baricitinib 8 mg daily. In dose-ranging Study II, there was not a clear trend of dose response, with similar response rates for 1 mg and 4 mg and 2 mg and 8 mg.
Confirmatory Studies
- The efficacy and safety of baricitinib 2 mg once daily was assessed in two confirmatory phase 3 trials. These trials were randomized, double-blind, multicenter studies in patients with active rheumatoid arthritis diagnosed according to American College of Rheumatology (ACR)/European League Against Rheumatism 2010 criteria. Patients over 18 years of age were eligible if at least 6 tender and 6 swollen joints were present at baseline. The two studies (Studies III and IV) evaluated baricitinib 2 mg and baricitinib 4 mg.
- Study III (NCT01721057) was a 24-week trial in 684 patients with moderately to severely active rheumatoid arthritis who had an inadequate response or intolerance to conventional DMARDs (cDMARDs). Patients received baricitinib 2 mg or 4 mg once daily or placebo added to existing background cDMARD treatment. From Week 16, non-responding patients could be rescued to receive baricitinib 4 mg once daily. The primary endpoint was the proportion of patients who achieved an ACR20 response at Week 12.
- Study IV (NCT01721044) was a 24-week trial in 527 patients with moderately to severely active rheumatoid arthritis who had an inadequate response or intolerance to 1 or more TNF inhibitor therapies with or without other biologic DMARDs (TNFi-IR). Patients received baricitinib 2 mg or baricitinib 4 mg once daily or placebo added to background cDMARD treatment. From Week 16, non-responding patients could be rescued to receive baricitinib 4 mg once daily. The primary endpoint was the proportion of patients who achieved an ACR20 response at Week 12.
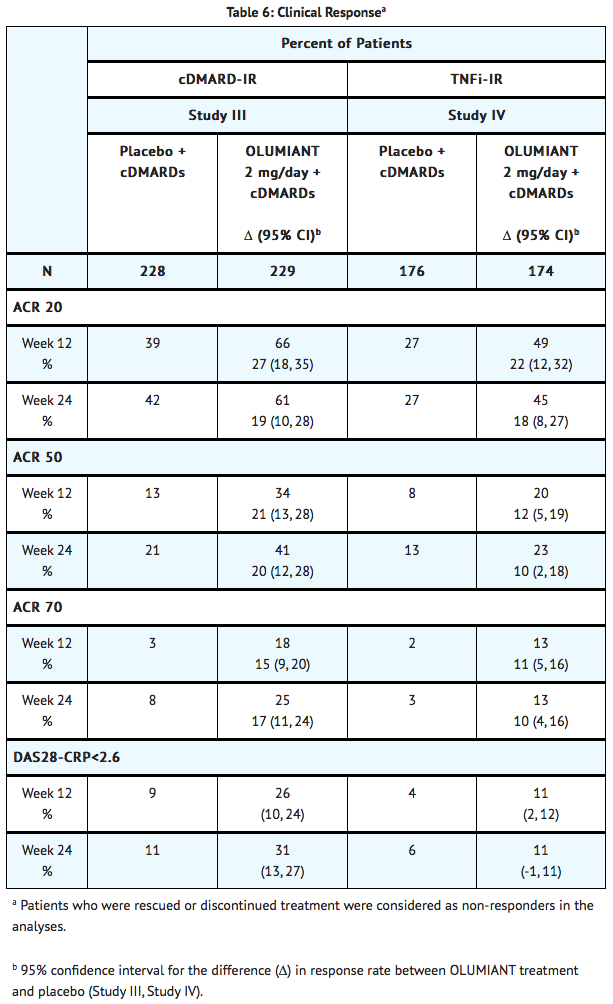
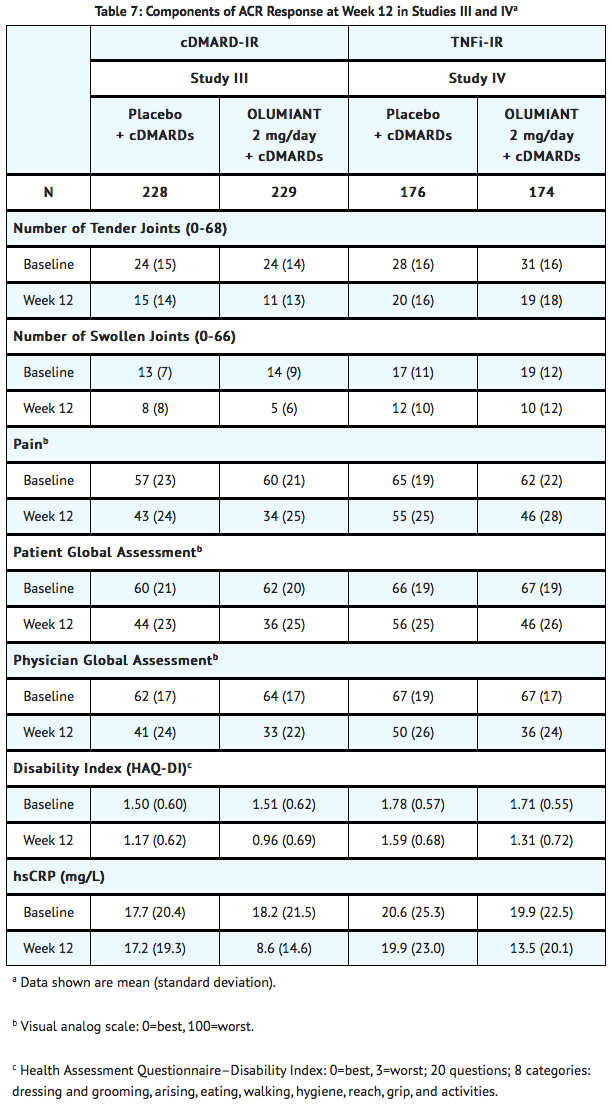
Clinical Response
- The percentages of baricitinib-treated patients achieving ACR20, ACR50, and ACR70 responses, and Disease Activity Score (DAS28-CRP) <2.6 in Studies III and IV are shown in TABLE 6.
- Patients treated with baricitinib had higher rates of ACR response and DAS28-CRP <2.6 versus placebo-treated patients at Week 12 (Studies III and IV) (TABLE 6).
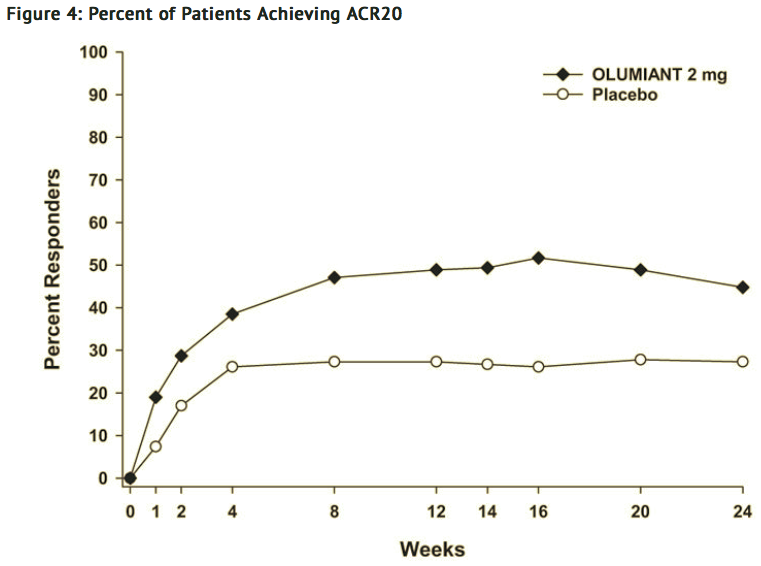
- In Study IV, higher ACR20 response rates (FIGURE 4) were observed as early as 1 week with baricitinib 2 mg versus placebo.
- In Study IV, the proportions of patients achieving DAS28-CRP <2.6 who had at least 3 active joints at the end of Week 24 were 18.2% and 10.5%, in the placebo and baricitinib 2 mg arms, respectively.
Physical Function Response
- Improvement in physical function was measured by the Health Assessment Questionnaire-Disability Index (HAQ-DI). Patients receiving baricitinib 2 mg demonstrated greater improvement from baseline in physical functioning compared to placebo at Week 24. The mean difference (95% CI) from placebo in HAQ-DI change from baseline at Week 24 was -0.24 (-0.35, -0.14) in Study III and -0.23 (-0.35, -0.12) in Study IV.
Other Health Related Outcomes
- General health status was assessed by the Short Form health survey (SF-36). In Studies III and IV, compared to placebo, patients treated with baricitinib 2 mg demonstrated greater improvement from baseline in the physical component summary (PCS) score and the physical function, role physical, bodily pain, vitality, and general health domains at Week 12, with no consistent improvements in the mental component summary (MCS) scores or the role emotional, mental health, and social functioning domains.
How Supplied
- Baricitinib for oral administration is available as debossed, film-coated, immediate-release tablets. Each tablet contains a recessed area on each face of the tablet surface.
Storage
- Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F).
- Keep out of reach of children.
Images
Drug Images
{{#ask: Page Name::Baricitinib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Baricitinib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Patient Counseling
- Advise patients of the potential benefits and risks of baricitinib.
Infections
- Inform patients that they may be more likely to develop infections when taking baricitinib. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of an infection.
- Advise patients that the risk of herpes zoster is increased in patients treated with baricitinib and some cases can be serious.
Malignancies and Lymphoproliferative Disorders
- Inform patients that baricitinib may increase their risk of certain cancers, and that lymphoma and other cancers have been observed in patients taking baricitinib. Instruct patients to inform their healthcare provider if they have ever had any type of cancer.
Thrombosis
- Advise patients that events of DVT and PE have been reported in clinical studies with baricitinib. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of a DVT or PE.
Laboratory Abnormalities
- Inform patients that baricitinib may affect certain lab tests, and that blood tests are required before and during baricitinib treatment.
Lactation
- Advise a woman not to breastfeed during treatment with baricitinib.
Medication Guide

Precautions with Alcohol
Alcohol-Baricitinib interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Baricitinib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.