Olaparib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Olaparib is an antineoplastic agent that is FDA approved for the treatment of patients with deleterious or suspected deleterious germline BRCA mutated (as detected by an FDA-approved test) advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy. Common adverse reactions include cough, constipation, dysgeusia, peripheral edema, back pain, dizziness, headache, urinary tract infection, dyspnea, and rash. leukopenia, stomatitis, peripheral neuropathy, pyrexia, hypomagnesemia, hyperglycemia, anxiety, depression, insomnia, dysuria, urinary incontinence, dry skin/ eczema, pruritis, hypertension, venous thrombosis (including pulmonary embolism), and hot flush.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Advanced ovarian cancer
Treatment of gBRCA-mutated advanced ovarian cancer
- Olaparib is indicated as monotherapy in patients with deleterious or suspected deleterious germline BRCA mutated (as detected by an FDA-approved test) advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy.
- The indication is approved under accelerated approval based on objective response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Dosing Information
Patient Selection
- Select patients for the treatment of advanced ovarian cancer with Olaparib based on the presence of deleterious or suspected deleterious germline BRCA-mutations .
Recommended Dosing
- The recommended dose of Olaparib is 400 mg (eight 50 mg capsules) taken twice daily, for a total daily dose of 800 mg. Continue treatment until disease progression or unacceptable toxicity.
- If a patient misses a dose of Olaparib , instruct patients to take their next dose at its scheduled time.
- Swallow capsule whole. Do not chew, dissolve, or open capsule. Do not take capsules which appear deformed or show evidence of leakage .
Dose Adjustments for Adverse Reactions
- To manage adverse reactions, consider dose interruption of treatment or dose reduction.
- The recommended dose reduction is to 200 mg (four 50 mg capsules) taken twice daily, for a total daily dose of 400 mg.
- If a further final dose reduction is required, then reduce to 100 mg (two 50 mg capsules) taken twice daily, for a total daily dose of 200 mg.
Dose Modifications for Use with CYP3A Inhibitors
- Avoid concomitant use of strong and moderate CYP3A inhibitors and consider alternative agents with less CYP3A inhibition. If the inhibitor cannot be avoided, reduce the Olaparib dose to 150 mg (three 50 mg capsules) taken twice daily for a strong CYP3A inhibitor or 200 mg (four 50 mg capsules) taken twice daily for a moderate CYP3A inhibitor
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Olaparib in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Olaparib in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Olaparib in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Olaparib in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Olaparib in pediatric patients.
Contraindications
- None
Warnings
Myelodysplastic syndrome/Acute Myeloid Leukemia
- Myelodysplastic syndrome/Acute Myeloid Leukemia (MDS/AML) have been confirmed in 6 out of 298 (2%) patients enrolled in a single arm trial of Olaparib monotherapy, in patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) advanced cancers. In a randomized placebo controlled trial, MDS/AML occurred in 3 out of 136 (2%) patients with advanced ovarian cancer treated with Olaparib . Overall, MDS/AML were reported in 22 of 2,618 (<1%) patients treated with Olaparib . The majority of MDS/AML cases (17 of 22 cases) were fatal, and the duration of therapy with Olaparib in patients who developed secondary MDS/cancer-therapy related AML varied from <6 months to >2 years. All patients had previous chemotherapy with platinum agents and/or other DNA damaging agents.
- Monitor complete blood count testing at baseline and monthly thereafter. Do not start Olaparib until patients have recovered from hematological toxicity caused by previous chemotherapy (≤CTCAE Grade 1). For prolonged hematological toxicities, interrupt Olaparib and monitor blood counts weekly until recovery. If the levels have not recovered to CTCAE Grade 1 or less after 4 weeks, refer the patient to a hematologist for further investigations, including bone marrow analysis and blood sample for cytogenetics. If MDS/AML is confirmed, discontinue Olaparib .
Pneumonitis
- Pneumonitis, including fatal cases, occurred in <1% of patients treated with Olaparib . If patients present with new or worsening respiratory symptoms such as dyspnea, fever, cough, wheezing, or a radiological abnormality occurs, interrupt treatment with Olaparib and initiate prompt investigation. If pneumonitis is confirmed, discontinue Olaparib .
Embryo-Fetal Toxicity
- Olaparib can cause fetal harm when administered to a pregnant woman based on its mechanism of action and findings in animals. Olaparib was teratogenic and caused embryo-fetal toxicity in rats at exposures below those in patients receiving the recommended human dose of 400 mg twice daily. If the patient becomes pregnant while taking this drug, apprise the patient of the potential hazard to a fetus .
- Advise females of reproductive potential to avoid becoming pregnant while taking Olaparib . If contraceptive methods are being considered, use effective contraception during treatment and for at least one month after receiving the last dose of Olaparib
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions are discussed elsewhere in the labeling:
Clinical Trial Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- Olaparib 400 mg twice daily as monotherapy, has been studied in 300 patients with gBRCA-mutated advanced ovarian cancer, and 223 of these patients had received 3 or more prior lines of chemotherapy.
- In the 223 patients with gBRCA-mutated ovarian cancer who received 3 or more prior lines of chemotherapy (including 137 patients in Study 1 with measureable disease) , adverse reactions led to dose interruption in 40% of patients, dose reduction in 4%, and discontinuation in 7%. There were 8 (4%) patients with adverse reactions leading to death, two were attributed to acute leukemia, and one each was attributed to COPD, cerebrovascular accident, intestinal perforation, pulmonary embolism, sepsis, and suture rupture. Table 1 presents the frequency of adverse reactions reported in ≥20% of 223 patients (in 6 studies) with gBRCA-mutated advanced ovarian cancer who had received 3 or more prior lines of chemotherapy who were treated with Olaparib 400 mg twice daily. The median exposure to Olaparib in these patients was 158 days.
Hematologic and Lymphatic
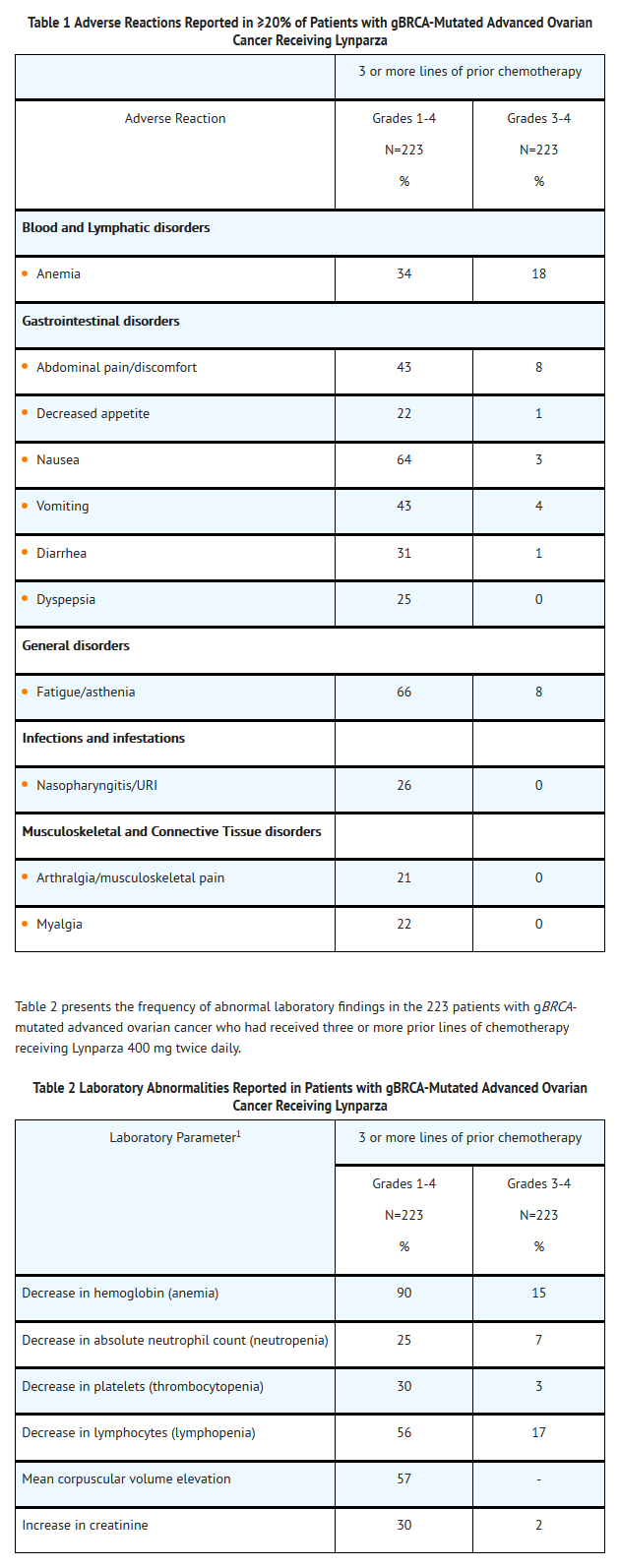
- The following adverse reactions and laboratory abnormalities have been identified in ≥10 to <20% of the 223 patients receiving Olaparib and not included in the table: cough, constipation, dysgeusia, peripheral edema, back pain, dizziness, headache, urinary tract infection, dyspnea, and rash. The following adverse reactions and laboratory abnormalities have been identified in ≥1 to <10% of the 223 patients receiving Olaparib and not included in the table: leukopenia, stomatitis, peripheralneuropathy, pyrexia, hypomagnesemia, hyperglycemia, anxiety, depression, insomnia, dysuria, urinary incontinence, vulvovaginal disorder, dry skin/ eczema, pruritis, hypertension, venous thrombosis (including pulmonary embolism), and hot flush.
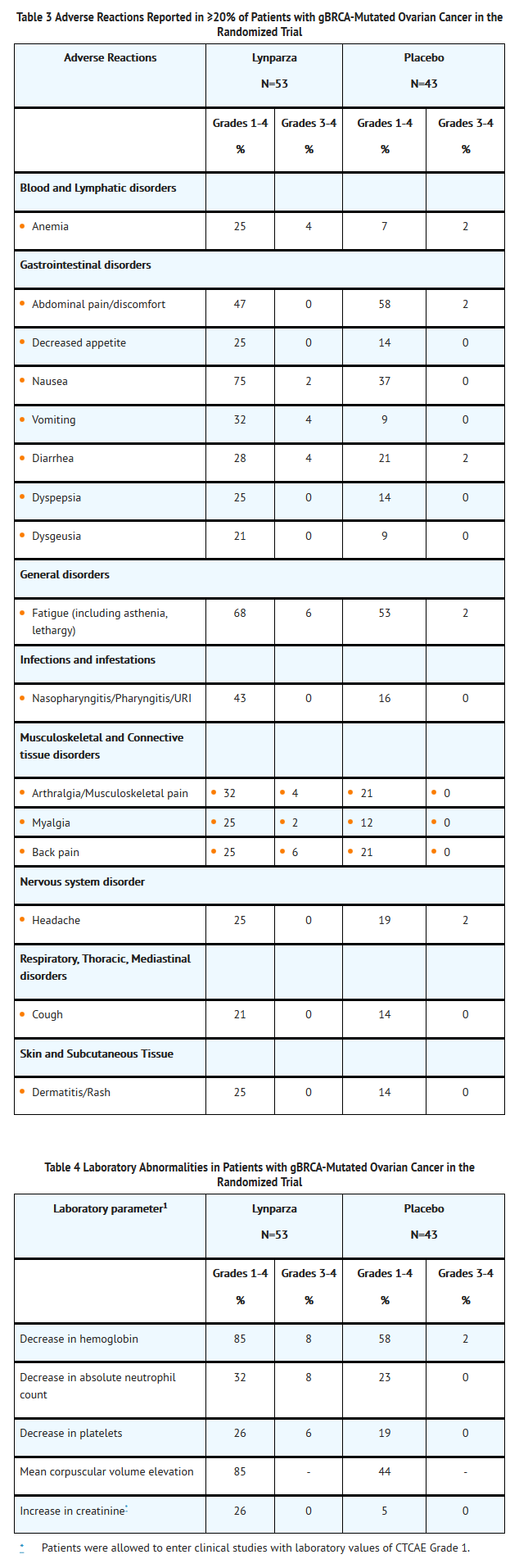
- Table 3 presents adverse reactions reported in ≥20% of patients from a randomized trial of Olaparib 400 mg twice daily as maintenance monotherapy compared to placebo in patients with platinum sensitive, relapsed, high-grade serous ovarian cancer following treatment with 2 or more platinum-containing regimens. Table 4 presents the laboratory abnormalities in patients from this randomized trial. Of the 96 patients with gBRCA-mutation, 53 received Olaparib , and 43 received placebo. The median duration on treatment with Olaparib was 11.1 months for patients with a gBRCA mutation compared to 4.4 months for patients with gBRCA mutation on placebo.
- Adverse reactions led to dose interruptions in 26% of those receiving Olaparib and 7% of those receiving placebo; dose reductions in 15% of Olaparib and 5% of placebo patients; and discontinuation in 9% of Olaparib and 0% in placebo patients. One (2%) patient on Olaparib died as a result of an adverse reaction.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Olaparib in the drug label.
Drug Interactions
- Olaparib is primarily metabolized by CYP3A.
Anticancer Agents
- Clinical studies of Olaparib in combination with other myelosuppressive anticancer agents, including DNA damaging agents, indicate a potentiation and prolongation of myelosuppressive toxicity.
Drugs that may Increase Olaparib Plasma Concentrations
- In patients (N=57), co-administration of itraconazole, a strong CYP3A inhibitor, increased AUC of olaparib by 2.7-fold. A moderate CYP3A inhibitor, fluconazole, is predicted to increase the AUC of olaparib by 2-fold.
- Avoid concomitant use of strong CYP3A inhibitors (e.g., itraconazole, telithromycin, clarithromycin, ketoconazole, voriconazole, nefazodone, posaconazole, ritinovir, lopinavir/ritinovir, indinavir, saquinavir, nelfinavir, boceprevir, telaprevir) and moderate CYP3A inhibitors (e.g., amprenavir, aprepitant, atazanavir, ciprofloxacin, crizotinib, darunavir/ritonavir, diltiazem, erythromycin, fluconazole, fosamprenavir, imatinib, verapamil). If the strong or moderate CYP3A inhibitors must be co-administered, reduce the dose of Olaparib .
- Avoid grapefruit and Seville oranges during Olaparib treatment .
Drugs that may Decrease Olaparib Plasma Concentrations
- In patients (N=22), co-administration of rifampicin, a strong CYP3A inducer, decreased AUC of olaparib by 87%. A moderate CYP3A inducer, efavirenz, is predicted to decrease the AUC of olaparib by 50-60%.
- Avoid concomitant use of strong CYP3A inducers (e.g., phenytoin, rifampicin, carbamazepine, St. John’s Wort) and moderate CYP3A4 inducers (e.g., bosentan, efavirenz, etravirine, modafinil, nafcillin). If a moderate CYP3A inducer cannot be avoided, be aware of a potential for decreased efficacy of Olaparib
Use in Specific Populations
Pregnancy
Risk summary
- Olaparib can cause fetal harm when administered to a pregnant woman based on its mechanism of action and findings in animals. Olaparib was teratogenic and caused embryo-fetal toxicity in rats at exposures below those in patients receiving the recommended human dose of 400 mg twice daily. If this drug is used during pregnancy, or if a patient becomes pregnant while taking this drug, apprise the patient of the potential hazard to the fetus and the potential risk for loss of the pregnancy.
Animal Data
- In a fertility and early embryonic development study in female rats, olaparib was administered orally for 14 days before mating through to day 6 of pregnancy, which resulted in increased post-implantation loss at a dose level of 15 mg/kg/day (with maternal systemic exposures approximately 11% of the human exposure (AUC0-24h) at the recommended dose).
- In an embryo-fetal development study, pregnant rats received oral doses of 0.05 and 0.5 mg/kg/day olaparib during the period of organogenesis. A dose of 0.5 mg/kg/day (with maternal systemic exposures approximately 0.3% of human exposure (AUC0-24h) at the recommended dose) caused embryo-fetal toxicities including increased post-implantation loss and major malformations of the eyes (anophthalmia, microphthalmia), vertebrae/ribs (extra rib or ossification center; fused or absent neural arches, ribs, and sternebrae), skull (fused exoccipital) and diaphragm (hernia). Additional abnormalities or variants included incomplete or absent ossification (vertebrae/sternebrae, ribs, limbs) and other findings in the vertebrae/sternebrae, pelvic girdle, lung, thymus, liver, ureter and umbilical artery. Some findings noted above in the eyes, ribs and ureter were observed at a dose of 0.05 mg/kg/day olaparib at lower incidence.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Olaparib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Olaparib during labor and delivery.
Nursing Mothers
- It is not known whether olaparib is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from olaparib, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and efficacy of Olaparib has not been established in pediatric patients.
Geriatic Use
- In clinical studies of Olaparib enrolling 735 patients with advanced solid tumors [the majority (69%) of whom had ovarian cancer] who received Olaparib 400 mg twice daily as monotherapy, 148 (20%) of patients were aged ≥65 years. The safety profile was similar irrespective of age with the exception of AEs of CTCAE ≥3 which were reported more frequently in patients aged ≥65 years (53.4%) than those <65 years (43.4%). No individual adverse event or System Organ Class accounted for this observed difference.
Gender
There is no FDA guidance on the use of Olaparib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Olaparib with respect to specific racial populations.
Renal Impairment
- Based on preliminary data, a 1.5 fold increase in mean exposure (AUC) was observed in patients with mild renal impairment (CLcr = 50-80 mL/min) compared to patients with normal renal function (CLcr >80 mL/min). No dose adjustment to the starting dose is required in patients with CLcr of 50 to 80 mL/min, but patients should be monitored closely for toxicity. There are no data in patients with moderate or severe renal impairment (CLcr <50 mL/min) or patients on dialysis
Hepatic Impairment
- The effect of hepatic impairment on exposure to Olaparib has not been studied. Patients with bilirubin >1.5 X ULN and AST/ALT ≥2.5 X ULN (≥5 X ULN in the presence of liver metastases) were excluded from Olaparib clinical trials. There are no data in patients with baseline hepatic impairment (serum bilirubin >1.5 X ULN)
Females of Reproductive Potential and Males
- Olaparib can cause fetal harm when administered to a pregnant woman . Advise female patients of reproductive potential to avoid pregnancy while taking Olaparib . If contraceptive methods are being considered, use highly effective contraception during treatment with Olaparib and for at least one month following the last dose of Olaparib . Instruct patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking Olaparib .
Immunocompromised Patients
There is no FDA guidance one the use of Olaparib in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Olaparib in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Olaparib in the drug label.
Overdosage
- There is no specific treatment in the event of Olaparib overdose, and symptoms of overdose are not established. In the event of an overdose, physicians should follow general supportive measures and should treat symptomatically.
Pharmacology
Mechanism of Action
- Olaparib is an inhibitor of poly (ADP-ribose) polymerase (PARP) enzymes, including PARP1, PARP2, and PARP3. PARP enzymes are involved in normal cellular homeostasis, such as DNA transcription, cell cycle regulation, and DNA repair. Olaparib has been shown to inhibit growth of select tumor cell linesin vitro and decrease tumor growth in mouse xenograft models of human cancer both as monotherapy or following platinum-based chemotherapy. Increased cytotoxicity and anti-tumor activity following treatment with olaparib were noted in cell lines and mouse tumor models with deficiencies in BRCA. In vitro studies have shown that olaparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and increased formation ofPARP-DNA complex, resulting in disruption of cellular homeostasis and cell death.
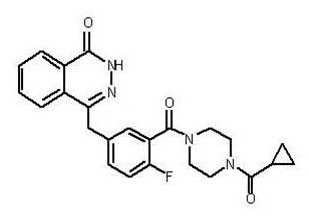
Structure
- Olaparib is an inhibitor of the mammalian polyadenosine 5’-diphosphoribose polymerase (PARP) enzyme.
The chemical name is 4-[(3-{[4-(cyclopropylcarbonyl)piperazin-1-yl]carbonyl}-4-fluorophenyl)methyl]phthalazin-1(2H)-one and it has the following chemical structure:
- The empirical molecular formula for Olaparib is C24H23FN4O3 and the relative molecular mass is 434.46.
- Olaparib is a crystalline solid, is non-chiral and shows pH-independent low solubility of approximately 0.1 mg/mL across the physiological pH range.
- Olaparib is available in 50 mg capsules for oral administration. Each capsule contains olaparib as the active ingredient and the following inactive ingredients:
- Capsule content: lauroyl polyoxylglycerides
- Capsule shell: hypromellose, titanium dioxide, gellan gum, potassium acetate
- Capsule printing ink: shellac, ferrosoferric oxide
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Olaparib in the drug label.
Pharmacokinetics
Absorption
- Following oral administration of olaparib via the capsule formulation, absorption is rapid with peak plasma concentrations typically achieved between 1 to 3 hours after dosing. On multiple dosing there is no marked accumulation (accumulation ratio of 1.4 – 1.5 for twice daily dosing), with steady state exposures achieved within 3 to 4 days.
- Limited data suggest that the systemic exposure (AUC) of olaparib increases less than proportionally with dose over the dose range of 100 to 400 mg, but the PK data were variable across trials.
- Co-administration with a high fat meal slowed the rate (Tmax delayed by 2 hours) of absorption, but did not significantly alter the extent of olaparib absorption (mean AUC increased by approximately 20%).
Distribution
- Olaparib had a mean (± standard deviation) apparent volume of distribution at steady state of 167 ± 196 L after a single 400 mg dose of olaparib. The in vitro protein binding of olaparib at plasma concentrations achieved following dosing at 400 mg twice daily is approximately 82%.
Metabolism
- In vitro, CYP3A4 was shown to be the enzyme primarily responsible for the metabolism of olaparib.
- Following oral dosing of 14C-olaparib to female patients, unchanged olaparib accounted for the majority of the circulating radioactivity in plasma (70%). It was extensively metabolized with unchanged drug accounting for 15% and 6% of radioactivity in urine and feces, respectively. The majority of the metabolism is attributable to oxidation reactions with a number of the components produced undergoing subsequent glucuronide or sulfate conjugation.
Excretion
- A mean (± standard deviation) terminal plasma half-life of 11.9 ± 4.8 hours and apparent plasma clearance of 8.6 ± 7.1L/h were observed after a single 400 mg dose of olaparib.
- Following a single dose of 14C-olaparib, 86% of the dosed radioactivity was recovered within a 7-day collection period, 44% via the urine and 42% via the feces. The majority of the material was excreted as metabolites.
- Based on preliminary data from a dedicated renal impairment trial, the mean AUC and Cmax of olaparib increased by 1.5- and 1.2-fold, respectively, when olaparib was dosed in patients with mild renal impairment (CLcr = 50-80 mL/min; N=14) compared to those with normal renal function (CLcr >80 mL/min; N=8). There are no data in patients with CLcr <50 mL/min or in patients on dialysis.
Drug Interactions
- In vitro, olaparib was an inhibitor of CYP3A4 and an inducer of CYP2B6 at higher concentrations than are clinically achieved. Olaparib produced little/no inhibition of other CYP isozymes. In vitro studies have shown that olaparib is a substrate of CYP3A4.
- Based on the data from a drug-interaction trial (N=57), the AUC and Cmax of olaparib increased by 2.7-and 1.4-fold, respectively, when olaparib was administered in combination with itraconazole, a strong CYP3A inhibitor. Simulations using physiologically-based pharmacokinetic (PBPK) models suggested that a moderate CYP3A inhibitor (fluconazole) may increase the AUC and Cmax of olaparib by 2-and 1.1-fold, respectively.
- Based on the data from a drug-interaction trial (N=22), the AUC and Cmax of olaparib decreased by 87% and 71%, respectively, when olaparib was administered in combination with rifampicin, a strong CYP3A inducer. Simulations using PBPK models suggested that a moderate CYP3A inducer (efavirenz) may decrease the AUC and Cmax of olaparib by 50 - 60% and 20 - 30%, respectively.
- In vitro studies have shown that olaparib is a substrate of P-gp and an inhibitor of BCRP, OATP1B1, OCT1, OCT2, OAT3, MATE1 and MATE2K. The clinical relevance of these findings is unknown.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity studies have not been conducted with olaparib.
- Olaparib was clastogenic in an in vitro chromosomal aberration assay in mammalian CHO cells and in an in vivo rat bone marrow micronucleus assay. This clastogenicity is consistent with genomic instability resulting from the primary pharmacology of olaparib and indicates potential for genotoxicity in humans. Olaparib was not mutagenic in a bacterial reverse mutation (Ames) test.
- In a fertility study, female rats received oral olaparib at doses of 0.05, 0.5, and 15 mg/kg/day for at least 14 days before mating through the first week of pregnancy. There were no adverse effects on mating and fertility rates at doses up to 15 mg/kg/day (maternal systemic exposures approximately 11% of the human exposure (AUC0-24h) at the recommended dose).
- In a male fertility study, olaparib had no effect on mating and fertility in rats at oral doses up to 40 mg/kg/day following at least 70 days of olaparib treatment (with systemic exposures of approximately 7% of the human exposure (AUC0-24h) at the recommended dose).
Clinical Studies
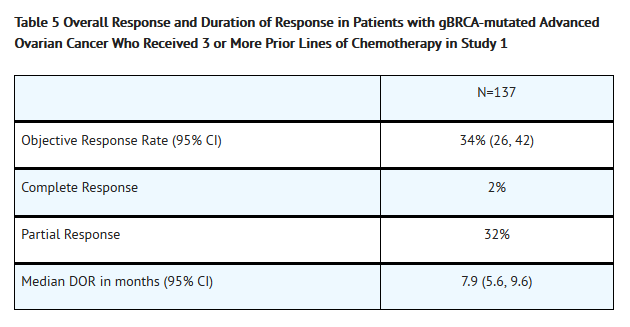
- The efficacy of Olaparib was investigated in a single-arm study in patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) advanced cancers (Study 1). A total of 137 patients with measurable, gBRCAm-associated ovarian cancer treated with three or more prior lines of chemotherapy were enrolled. All patients received Olaparib at a dose of 400 mg twice daily as monotherapy until disease progression or intolerable toxicity. Objective response rate (ORR) and duration of response (DOR) were assessed by the investigator according to RECIST v1.1.
- The median age of the patients was 58 years, the majority were Caucasian (94%) and 93% had an ECOG PS of 0 or 1. Deleterious or suspected deleterious, germline BRCA mutation status was verified retrospectively in 97% (59/61) of the patients for whom blood samples were available by the companion diagnostic BRACAnalysis CDx™, which is FDA approved for selection of patients for Olaparib treatment.
- Efficacy results from Study 1 are summarized in Table 5.
How Supplied
- Olaparib 50 mg is a white, opaque, hard capsule, marked in black ink with: “OLAPARIB 50 mg” on the cap and AstraZeneca logo on the body; available in:
- Bottles of 112 capsules NDC 0310-0657-58
Storage
- Store at 25ºC (77ºF), excursions permitted to 15-30ºC (59-86ºF) [see USP Controlled Room Temperature]
- Olaparib should not be exposed to temperatures greater than 40ºC or 104ºF. Do not take Olaparib if it is suspected of having been exposed to temperatures greater than 40ºC or 104ºF.
Images
Drug Images
{{#ask: Page Name::Olaparib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Olaparib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Dosing Instructions: Inform patients on how to take Olaparib . Olaparib should be taken twice daily. Instruct patients that if they miss a dose of Olaparib , not to take an extra dose to make up for the one that they missed. They should take their next normal dose at the usual time. Each capsule should be swallowed whole. Do not chew, dissolve, or open the capsule. Patient should not take Olaparib with grapefruit or Seville oranges.
- MDS/AML: Advise patients to contact their healthcare provider if they experience weakness, feeling tired, fever, weight loss, frequent infections, bruising, bleeding easily, breathlessness, blood in urine or stool, and/or laboratory findings of low blood cell counts, or a need for blood transfusions. This may be a sign of hematological toxicity or a more serious uncommon bone marrow problem called ‘myelodysplastic syndrome’ (MDS) or ‘acute myeloid leukemia’ (AML) which have been reported in patients treated with Olaparib .
- Pneumonitis: Advise patients to contact their healthcare provider if they experience any new or worsening respiratory symptoms including shortness of breath, fever, cough, or wheezing
- Pregnancy and Females of Reproductive Potential: Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of the pregnancy . Advise females of reproductive potential to use effective contraception during treatment with Olaparib and for at least one month after receiving the last dose of Olaparib .
- Nursing Mothers: Advise patients not to breastfeed while taking Olaparib .
- Nausea/vomiting: Advise patients that mild or moderate nausea and/or vomiting is very common in patients receiving Olaparib and that they should contact their healthcare provider who will advise on available antiemetic treatment options.
Precautions with Alcohol
- Alcohol-Olaparib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Lynparza ®
Look-Alike Drug Names
There is limited information regarding Olaparib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Olaparib
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Olaparib |Label Name=Olaparib05.png
}}
{{#subobject:
|Label Page=Olaparib |Label Name=Olaparib06.png
}}