Vinorelbine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning
See full prescribing information for complete Boxed Warning.
* Vinorelbine injection should be administered under the supervision of a physician experienced in the use of cancer chemotherapeutic agents. This product is for intravenous (IV) use only. Intrathecal administration of other vinca alkaloids has resulted in death. Syringes containing this product should be labeled "WARNING – FOR IV USE ONLY. FATAL if given intrathecally."
|
Overview
Vinorelbine is an antineoplastic that is FDA approved for the treatment of single agent or in combination with cisplatin for the first-line treatment of ambulatory patients with unresectable, advanced nonsmall cell lung cancer (NSCLC). There is a Black Box Warning for this drug as shown here. Common adverse reactions include alopecia, injection site reaction, diarrhea, nausea, vomiting, asthenia, neuromyopathy.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Vinorelbine Injection USP is indicated as a single agent or in combination with cisplatin for the first-line treatment of ambulatory patients with unresectable, advanced nonsmall cell lung cancer (NSCLC). In patients with Stage IV NSCLC, Vinorelbine Injection USP is indicated as a single agent or in combination with cisplatin. In Stage III NSCLC, Vinorelbine Injection USP is indicated in combination with cisplatin.
Single-Agent Vinorelbine Injection
- The usual initial dose of single-agent vinorelbine injection is 30 mg/m2 administered weekly. The recommended method of administration is an intravenous injection over 6 to 10 minutes. In controlled trials, single-agent vinorelbine injection was given weekly until progression or dose-limiting toxicity.
- Vinorelbine Injection in Combination with Cisplatin
- Vinorelbine injection may be administered weekly at a dose of 25 mg/m2 in combination with cisplatin given every 4 weeks at a dose of 100 mg/m2.
- Blood counts should be checked weekly to determine whether dose reductions of vinorelbine injection and/or cisplatin are necessary. In the SWOG study, most patients required a 50% dose reduction of vinorelbine injection at day 15 of each cycle and a 50% dose reduction of cisplatin by cycle 3.
- Vinorelbine injection may also be administered weekly at a dose of 30 mg/m2 in combination with cisplatin, given on days 1 and 29, then every 6 weeks at a dose of 120 mg/m2.
Dose Modifications for Vinorelbine Injection
- The dosage should be adjusted according to hematologic toxicity or hepatic insufficiency, whichever results in the lower dose for the corresponding starting dose of vinorelbine injection.
Dose Modifications for Hematologic Toxicity
- Granulocyte counts should be ≥1000 cells/mm3 prior to the administration of vinorelbine injection. Adjustments in the dosage of vinorelbine injection should be based on granulocyte counts obtained on the day of treatment according to TABLE 5.
- Dose Modifications for Concurrent Hematologic Toxicity and Hepatic =====Insufficiency=====
- In patients with both hematologic toxicity and hepatic insufficiency, the lower of the doses based on the corresponding starting dose of vinorelbine injection determined fromTABLE 5andTABLE 6should be administered.
Dose Modifications for Renal Insufficiency
- No dose adjustments for vinorelbine injection are required for renal insufficiency. Appropriate dose reductions for cisplatin should be made when vinorelbine injection is used in combination.
Dose Modifications for Neurotoxicity
- If grade ≥2 neurotoxicity develops, vinorelbine injection should be discontinued.
Administration Precautions
- Caution—vinorelbine injection must be administered intravenously. It is extremely important that the intravenous needle or catheter be properly positioned before any vinorelbine injection is injected. Leakage into surrounding tissue during intravenous administration of vinorelbine injection may cause considerable irritation, local tissue necrosis, and/or thrombophlebitis. If extravasation occurs, the injection should be discontinued immediately, and any remaining portion of the dose should then be introduced into another vein. Since there are no established guidelines for the treatment of extravasation injuries with vinorelbine injection, institutional guidelines may be used. The ONS Chemotherapy Guidelines provide additional recommendations for the prevention of extravasation injuries.1
- As with other toxic compounds, caution should be exercised in handling and preparing the solution of vinorelbine injection. Skin reactions may occur with accidental exposure. The use of gloves is recommended. If the solution of vinorelbine injection contacts the skin or mucosa, immediately wash the skin or mucosa thoroughly with soap and water. Severe irritation of the eye has been reported with accidental contamination of the eye with another vinca alkaloid. If this happens with vinorelbine injection, the eye should be flushed with water immediately and thoroughly.
- Procedures for proper handling and disposal of anticancer drugs should be used. Several guidelines on this subject have been published.2–8 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
- Vinorelbine injection is a clear, colorless to pale yellow solution. Parenteral drug products should be visually inspected for particulate matter and discoloration prior to administration whenever solution and container permit. If particulate matter is seen, vinorelbine injection should not be administered.
Preparation for Administration
- Vinorelbine injection must be diluted in either a syringe or IV bag using one of the recommended solutions. The diluted vinorelbine injection should be administered over 6 to 10 minutes into the side port of a free-flowing IV closest to the IV bag followed by flushing with at least 75 to 125 mL of one of the solutions. Diluted vinorelbine injection may be used for up to 24 hours under normal room light when stored in polypropylene syringes or polyvinyl chloride bags at 5° to 30°C (41° to 86°F)
Syringe
- The calculated dose of vinorelbine injection should be diluted to a concentration between 1.5 and 3 mg/mL. The following solutions may be used for dilution:
- 5% Dextrose Injection, USP
- 0.9% Sodium Chloride Injection, USP
IV Bag
- The calculated dose of vinorelbine injection should be diluted to a concentration between 0.5 and 2 mg/mL. The following solutions may be used for dilution:
- 5% Dextrose Injection, USP
- 0.9% Sodium Chloride Injection, USP
- 0.45% Sodium Chloride Injection, USP
- 5% Dextrose and 0.45% Sodium Chloride Injection, USP
- Ringer's Injection, USP
- Lactated Ringer's Injection, USP
Stability
- Unopened vials of vinorelbine injection are stable until the date indicated on the package when stored under refrigeration at 2° to 8°C (36° to 46°F) and protected from light in the carton. Unopened vials of vinorelbine injection are stable at temperatures up to 25°C (77°F) for up to 72 hours. This product should not be frozen.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vinorelbine in adult patients.
Non–Guideline-Supported Use
- Breast cancer[1]
- Carcinoma of cervix[2]
- Carcinoma of esophagus[3]
- Head and neck cancer[4]
- Hodgkin's disease[5]
- Kaposi's sarcoma[6]
- Malignant tumor of salivary gland[7]
- Non-Hodgkin's lymphoma[8]
- Non-small cell lung cancer[9]
- Ovarian cancer, Advanced, previously treated[10]
- Small cell carcinoma of lung[11]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Vinorelbine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vinorelbine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Vinorelbine in pediatric patients.
Contraindications
- Administration of vinorelbine tartrate injection is contraindicated in patients with pretreatment granulocyte counts <1000 cells/mm3
Warnings
|
Warning
See full prescribing information for complete Boxed Warning.
* Vinorelbine injection should be administered under the supervision of a physician experienced in the use of cancer chemotherapeutic agents. This product is for intravenous (IV) use only. Intrathecal administration of other vinca alkaloids has resulted in death. Syringes containing this product should be labeled "WARNING – FOR IV USE ONLY. FATAL if given intrathecally."
|
- Vinorelbine tartrate should be administered in carefully adjusted doses by or under the supervision of a physician experienced in the use of cancer chemotherapeutic agents.
- Patients treated with vinorelbine tartrate should be frequently monitored for myelosuppression both during and after therapy. Granulocytopenia is dose-limiting. Granulocyte nadirs occur between 7 and 10 days after dosing with granulocyte count recovery usually within the following 7 to 14 days.
- Complete blood counts with differentials should be performed and results reviewed prior to administering each dose of vinorelbine tartrate. Vinorelbine tartrate should not be administered to patients with granulocyte counts <1000 cells/mm3. Patients developing severe granulocytopenia should be monitored carefully for evidence of infection and/or fever.
- Acute shortness of breath and severe bronchospasm have been reported infrequently, following the administration of vinorelbine tartrate and other vinca alkaloids, most commonly when the vinca alkaloid was used in combination with mitomycin. These adverse events may require treatment with supplemental oxygen, bronchodilators, and/or corticosteroids, particularly when there is pre-existing pulmonary dysfunction.
- Reported cases of interstitial pulmonary changes and acute respiratory distress syndrome (ARDS), most of which were fatal, occurred in patients treated with single-agent vinorelbine tartrate. The mean time to onset of these symptoms after vinorelbine administration was 1 week (range 3 to 8 days). Patients with alterations in their baseline pulmonary symptoms or with new onset of dyspnea, cough, hypoxia, or other symptoms should be evaluated promptly.
- Vinorelbine tartrate has been reported to cause severe constipation (e.g., Grade 3 to 4), paralytic ileus, intestinal obstruction, necrosis, and/or perforation. Some events have been fatal.
Adverse Reactions
Clinical Trials Experience
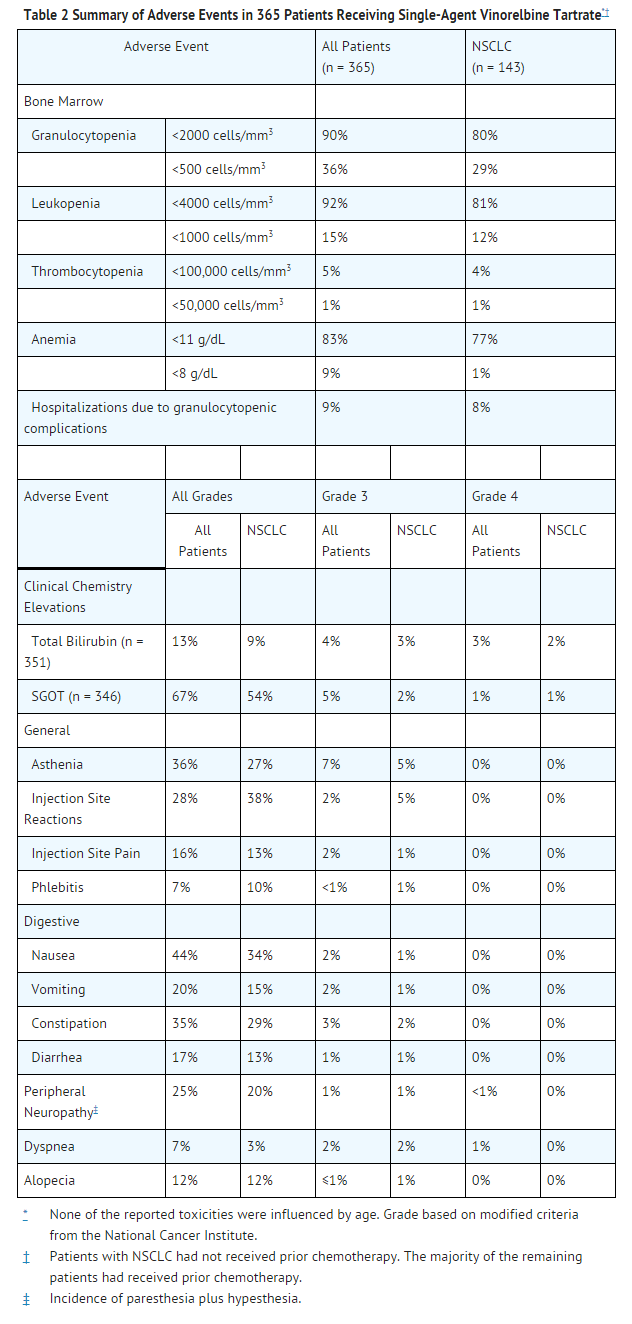
- The pattern of adverse reactions is similar whether vinorelbine tartrate is used as a single agent or in combination. Adverse reactions from studies with single-agent and combination use of vinorelbine tartrate are summarized in Tables 2 to 4.
Single-Agent Vinorelbine Tartrate
- Data in the following table are based on the experience of 365 patients (143 patients with NSCLC; 222 patients with advanced breast cancer) treated with IV vinorelbine tartrate as a single agent in 3 clinical studies. The dosing schedule in each study was 30 mg/m2 vinorelbine tartrate on a weekly basis.
Hematologic
- Granulocytopenia is the major dose-limiting toxicity with vinorelbine tartrate. Dose adjustments are required for hematologic toxicity and hepatic insufficiency. Granulocytopenia was generally reversible and not cumulative over time. Granulocyte nadirs occurred 7 to 10 days after the dose, with granulocyte recovery usually within the following 7 to 14 days. Granulocytopenia resulted in hospitalizations for fever and/or sepsis in 8% of patients. Septic deaths occurred in approximately 1% of patients. Prophylactic hematologic growth factors have not been routinely used with vinorelbine tartrate. If medically necessary, growth factors may be administered at recommended doses no earlier than 24 hours after the administration of cytotoxic chemotherapy. Growth factors should not be administered in the period 24 hours before the administration of chemotherapy.
- Whole blood and/or packed red blood cells were administered to 18% of patients who received vinorelbine tartrate.
Neurologic
- Loss of deep tendon reflexes occurred in less than 5% of patients. The development of severe peripheral neuropathy was infrequent (1%) and generally reversible.
Skin
- Like other anticancer vinca alkaloids, vinorelbine tartrate is a moderate vesicant. Injection site reactions, including erythema, pain at injection site, and vein discoloration, occurred in approximately one third of patients; 5% were severe. Chemical phlebitis along the vein proximal to the site of injection was reported in 10% of patients.
Gastrointestinal
- Prophylactic administration of antiemetics was not routine in patients treated with single-agent vinorelbine tartrate. Due to the low incidence of severe nausea and vomiting with single-agent vinorelbine tartrate, the use of serotonin antagonists is generally not required.
Hepatic
- Transient elevations of liver enzymes were reported without clinical symptoms.
Cardiovascular
- Chest pain was reported in 5% of patients. Most reports of chest pain were in patients who had either a history of cardiovascular disease or tumor within the chest. There have been rare reports of myocardial infarction.
Pulmonary
- Shortness of breath was reported in 3% of patients; it was severe in 2%. Interstitial pulmonary changes were documented.
Other
- Fatigue occurred in 27% of patients. It was usually mild or moderate but tended to increase with cumulative dosing.
- Other toxicities that have been reported in less than 5% of patients include jaw pain, myalgia, arthralgia, and rash. Hemorrhagic cystitis and the syndrome of inappropriate ADH secretion were each reported in <1% of patients.
Combination Use
- Adverse events for combination use are summarized inTABLES 3 and 4.
- Vinorelbine Tartrate in Combination with Cisplatin
- Vinorelbine Tartrate plus Cisplatin versus Single-Agent Cisplatin (Table 3)
- Myelosuppression was the predominant toxicity in patients receiving combination therapy, Grade 3 and 4 granulocytopenia of 82% compared to 5% in the single-agent cisplatin arm. Fever and/or sepsis related to granulocytopenia occurred in 11% of patients on vinorelbine tartrate and cisplatin compared to 0% on the cisplatin arm.
- Four patients on the combination died of granulocytopenia-related sepsis.
- During this study, the use of granulocyte colony-stimulating factor ([G-CSF] filgrastim) was permitted, but not mandated, after the first course of treatment for patients who experienced Grade 3 or 4 granulocytopenia (≤1000 cells/mm3) or in those who developed neutropenic fever between cycles of chemotherapy. Beginning 24 hours after completion of chemotherapy, G-CSF was started at a dose of 5 mcg/kg per day and continued until the total granulocyte count was >1000 cells/mm3 on 2 successive determinations. G-CSF was not administered on the day of treatment.
- Grade 3 and 4 anemia occurred more frequently in the combination arm compared to control, 24% vs. 8%, respectively. Thrombocytopenia occurred in 6% of patients treated with vinorelbine tartrate plus cisplatin compared to 2% of patients treated with cisplatin.
- The incidence of severe non-hematologic toxicity was similar among the patients in both treatment groups. Patients receiving vinorelbine tartrate plus cisplatin compared to single-agent cisplatin experienced more Grade 3 and/or 4 peripheral numbness (2% vs. <1%), phlebitis/thrombosis/embolism (3% vs. <1%), and infection (6% vs. <1%). Grade 3 to 4-constipation and/or ileus occurred in 3% of patients treated with combination therapy and in 1% of patients treated with cisplatin.
- Seven deaths were reported on the combination arm; 2 were related to cardiac ischemia, 1 massive cerebrovascular accident, 1 multisystem failure due to an overdose of vinorelbine tartrate, and 3 from febrile neutropenia. One death, secondary to respiratory infection unrelated to granulocytopenia, occurred with single-agent cisplatin.
- Vinorelbine Tartrate plus Cisplatin versus Vindesine plus Cisplatin versus Single-Agent Vinorelbine Tartrate (Table 4)
- Myelosuppression, specifically Grade 3 and 4 granulocytopenia, was significantly greater with the combination of vinorelbine tartrate plus cisplatin (79%) than with either single-agent vinorelbine tartrate (53%) or vindesine plus cisplatin (48%), P<0.0001. Hospitalization due to documented sepsis occurred in 4.4% of patients treated with vinorelbine tartrate plus cisplatin; 2% of patients treated with vindesine and cisplatin, and 4% of patients treated with single-agent vinorelbine tartrate. Grade 3 and 4 thrombocytopenia was infrequent in patients receiving combination chemotherapy and no events were reported with single-agent vinorelbine tartrate.
- The incidence of Grade 3 and/or 4 nausea and vomiting, alopecia, and renal toxicity were reported more frequently in the cisplatin-containing combinations compared to single-agent vinorelbine tartrate. Severe local reactions occurred in 2% of patients treated with combinations containing vinorelbine tartrate; none were observed in the vindesine plus cisplatin arm. Grade 3 and 4 neurotoxicity was significantly more frequent in patients receiving vindesine plus cisplatin (17%) compared to vinorelbine tartrate plus cisplatin (7%) and single-agent vinorelbine tartrate (9%) (P< 0.005). Cisplatin did not appear to increase the incidence of neurotoxicity observed with single-agent vinorelbine tartrate.
Postmarketing Experience
- In addition to the adverse events reported from clinical trials, the following events have been identified during post-approval use of vinorelbine tartrate. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to vinorelbine tartrate.
- Body as a Whole
- Systemic allergic reactions reported as anaphylaxis, pruritus, urticaria, and angioedema; flushing; and radiation recall events such as dermatitis and esophagitis have been reported.
- Hematologic
- Thromboembolic events, including pulmonary embolus and deep venous thrombosis, have been reported primarily in seriously ill and debilitated patients with known predisposing risk factors for these events.
- Neurologic
- Peripheral neurotoxicities such as, but not limited to, muscle weakness and disturbance of gait, have been observed in patients with and without prior symptoms. There may be increased potential for neurotoxicity in patients with pre-existing neuropathy, regardless of etiology, who receive vinorelbine tartrate. Vestibular and auditory deficits have been observed with vinorelbine tartrate, usually when used in combination with cisplatin.
- Skin
- Injection site reactions, including localized rash and urticaria, blister formation, and skin sloughing have been observed in clinical practice. Some of these reactions may be delayed in appearance.
- Gastrointestinal
- Dysphagia, mucositis, and pancreatitis have been reported.
- Cardiovascular
- Hypertension, hypotension, vasodilation, tachycardia, and pulmonary edema have been reported.
- Pulmonary
- Pneumonia has been reported.
- Musculoskeletal
- Headache has been reported, with and without other musculoskeletal aches and pains.
- Other
- Pain in tumor-containing tissue, back pain, and abdominal pain have been reported. Electrolyte abnormalities, including hyponatremia with or without the syndrome of inappropriate ADH secretion, have been reported in seriously ill and debilitated patients.
- Combination Use
- Patients with prior exposure to paclitaxel and who have demonstrated neuropathy should be monitored closely for new or worsening neuropathy. Patients who have experienced neuropathy with previous drug regimens should be monitored for symptoms of neuropathy while receiving vinorelbine tartrate. Vinorelbine tartrate may result in radiosensitizing effects with prior or concomitant radiation therapy.
Drug Interactions
There is limited information regarding Vinorelbine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Vinorelbine tartrate may cause fetal harm if administered to a pregnant woman. A single dose of vinorelbine has been shown to be embryo- and/or fetotoxic in mice and rabbits at doses of 9 mg/m2 and 5.5 mg/m2, respectively (one third and one sixth the human dose). At nonmaternotoxic doses, fetal weight was reduced and ossification was delayed. There are no studies in pregnant women. If vinorelbine tartrate is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant during therapy with vinorelbine tartrate.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Vinorelbine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Vinorelbine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Vinorelbine with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Vinorelbine with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Vinorelbine with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Vinorelbine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Vinorelbine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Vinorelbine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Vinorelbine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Vinorelbine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Vinorelbine in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
- Patients with prior exposure to paclitaxel and who have demonstrated neuropathy should be monitored closely for new or worsening neuropathy. Patients who have experienced neuropathy with previous drug regimens should be monitored for symptoms of neuropathy while receiving vinorelbine tartrate.
Patients treated with vinorelbine tartrate should be frequently monitored for myelosuppression both during and after therapy
- Patients developing severe granulocytopenia should be monitored carefully for evidence of infection and/or fever.
IV Compatibility
There is limited information regarding IV Compatibility of Vinorelbine in the drug label.
Overdosage
- There is no known antidote for overdoses of vinorelbine tartrate. Overdoses involving quantities up to 10 times the recommended dose (30 mg/m2) have been reported. The toxicities described were consistent with those listed in theADVERSE REACTIONSsection including paralytic ileus, stomatitis, and esophagitis. Bone marrow aplasia, sepsis, and paresis have also been reported.
- Fatalities have occurred following overdose of vinorelbine tartrate. If overdosage occurs, general supportive measures together with appropriate blood transfusions, growth factors, and antibiotics should be instituted as deemed necessary by the physician.
Pharmacology
There is limited information regarding Vinorelbine Pharmacology in the drug label.
Mechanism of Action
- Vinorelbine is a vinca alkaloid that interferes with microtubule assembly. The vinca alkaloids are structurally similar compounds comprised of 2 multiringed units, vindoline and catharanthine. Unlike other vinca alkaloids, the catharanthine unit is the site of structural modification for vinorelbine.
- The antitumor activity of vinorelbine is thought to be due primarily to inhibition of mitosis at metaphase through its interaction with tubulin. Like other vinca alkaloids, vinorelbine may also interfere with: 1) amino acid, cyclic AMP, and glutathione metabolism, 2) calmodulin-dependent Ca++-transport ATPase activity, 3) cellular respiration, and 4) nucleic acid and lipid biosynthesis. In intact tectal plates from mouse embryos, vinorelbine, vincristine, and vinblastine inhibited mitotic microtubule formation at the same concentration (2 µM), inducing a blockade of cells at metaphase.
- Vincristine produced depolymerization of axonal microtubules at 5 µM, but vinblastine and vinorelbine did not have this effect until concentrations of 30 µM and 40 µM, respectively. These data suggest relative selectivity of vinorelbine for mitotic microtubules.
Structure
- Vinorelbine Injection USP is for intravenous administration. Each vial contains vinorelbine tartrate, USP equivalent to 10 mg (1-mL vial) or 50 mg (5-mL vial) vinorelbine in Water for Injection. No preservatives or other additives are present. The aqueous solution is sterile and nonpyrogenic.
- Vinorelbine tartrate, USP is a semi-synthetic vinca alkaloid with antitumor activity. The chemical name is 3',4'-didehydro-4'-deoxy-C'-norvincaleukoblastine [R-(R*,R*)-2,3-dihydroxybutanedioate (1:2)(salt)].
- Vinorelbine tartrate, USP has the following structure:
- Vinorelbine tartrate, USP is a white to yellow or light brown amorphous powder. The aqueous solubility is >1000 mg/mL in distilled water. The pH of Vinorelbine Injection USP is approximately 3.5.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Vinorelbine in the drug label.
Pharmacokinetics
- The pharmacokinetics of vinorelbine were studied in 49 patients who received doses of 30 mg/m2 in 4 clinical trials. Doses were administered by 15- to 20-minute constant-rate infusions. Following intravenous administration, vinorelbine concentration in plasma decays in a triphasic manner.
- The initial rapid decline primarily represents distribution of drug to peripheral compartments followed by metabolism and excretion of the drug during subsequent phases. The prolonged terminal phase is due to relatively slow efflux of vinorelbine from peripheral compartments. The terminal phase half-life averages 27.7 to 43.6 hours and the mean plasma clearance ranges from 0.97 to 1.26 L/h per kg. Steady-state volume of distribution (Vss) values range from 25.4 to 40.1 L/kg.
- Vinorelbine demonstrated high binding to human platelets and lymphocytes. The free fraction was approximately 0.11 in pooled human plasma over a concentration range of 234 to 1169 ng/mL. The binding to plasma constituents in cancer patients ranged from 79.6% to 91.2%. Vinorelbine binding was not altered in the presence of cisplatin, 5-fluorouracil, or doxorubicin.
- Vinorelbine undergoes substantial hepatic elimination in humans, with large amounts recovered in feces after intravenous administration to humans. Two metabolites of vinorelbine have been identified in human blood, plasma, and urine; vinorelbine N-oxide and deacetylvinorelbine. Deacetylvinorelbine has been demonstrated to be the primary metabolite of vinorelbine in humans, and has been shown to possess antitumor activity similar to vinorelbine.
- Therapeutic doses of vinorelbine tartrate (30 mg/m2) yield very small, if any, quantifiable levels of either metabolite in blood or urine. The metabolism of vinca alkaloids has been shown to be mediated by hepatic cytochrome P450 isoenzymes in the CYP3A subfamily. This metabolic pathway may be impaired in patients with hepatic dysfunction or who are taking concomitant potent inhibitors of these isoenzymes.
- The effects of renal or hepatic dysfunction on the disposition of vinorelbine have not been assessed, but based on experience with other anticancer vinca alkaloids, dose adjustments are recommended for patients with impaired hepatic function.
- The disposition of radiolabeled vinorelbine given intravenously was studied in a limited number of patients. Approximately 18% and 46% of the administered dose was recovered in the urine and in the feces, respectively. Incomplete recovery in humans is consistent with results in animals where recovery is incomplete, even after prolonged sampling times. A separate study of the urinary excretion of vinorelbine using specific chromatographic analytical methodology showed that 10.9% ± 0.7% of a 30 mg/m2 intravenous dose was excreted unchanged in the urine.
- The influence of age on the pharmacokinetics of vinorelbine was examined using data from 44 cancer patients (average age, 56.7 ± 7.8 years; range, 41 to 74 years; with 12 patients ≥60 years and 6 patients ≥65 years) in 3 studies. CL (the mean plasma clearance), t1/2 (the terminal phase half-life), and VZ (the volume of distribution during terminal phase) were independent of age. A separate pharmacokinetic study was conducted in 10 elderly patients with metastatic breast cancer (age range, 66 to 81 years; 3 patients >75 years; normal liver function tests) receiving vinorelbine 30 mg/m2 intravenously. CL, Vss, and t1/2 were similar to those reported for younger adult patients in previous studies. No relationship between age, systemic exposure (AUCo-∞), and hematological toxicity was observed.
- The pharmacokinetics of vinorelbine are not influenced by the concurrent administration of cisplatin with vinorelbine tartrate.
Clinical Trials
- Data from 1 randomized clinical study (211 evaluable patients) with single-agent vinorelbine tartrate and 2 randomized clinical trials (1044 patients) using vinorelbine tartrate combined with cisplatin support the use of vinorelbine tartrate in patients with advanced nonsmall cell lung cancer (NSCLC).
Single-Agent Vinorelbine Tartrate
- Single-agent vinorelbine tartrate was studied in a North American, randomized clinical trial in which patients with Stage IV NSCLC, no prior chemotherapy, and Karnofsky Performance Status ≥70 were treated with vinorelbine tartrate (30 mg/m2) weekly or 5-fluorouracil (5-FU) (425 mg/m2 IV bolus) plus leucovorin (LV) (20 mg/m2 IV bolus) daily for 5 days every 4 weeks. A total of 211 patients were randomized at a 2:1 ratio to vinorelbine tartrate (143) or 5-FU/LV (68). Vinorelbine tartrate showed improved survival time compared to 5-FU/LV. In an intent-to-treat analysis, the median survival time was 30 weeks versus 22 weeks for patients receiving vinorelbine tartrate versus 5-FU/LV, respectively (P = 0.06). The 1-year survival rates were 24% (±4% SE) for vinorelbine tartrate and 16% (±5% SE) for the 5-FU/LV group, using the Kaplan-Meier product-limit estimates. The median survival time with 5-FU/LV was similar to or slightly better than that usually observed in untreated patients with advanced NSCLC, suggesting that the difference was not related to some unknown detrimental effect of 5-FU/LV therapy.
- The response rates (all partial responses) for vinorelbine tartrate and 5-FU/LV were 12% and 3%, respectively.
- Vinorelbine Tartrate in Combination with Cisplatin: Vinorelbine Tartrate plus Cisplatin versus Single-Agent Cisplatin
- A Phase III open-label, randomized study was conducted which compared vinorelbine tartrate (25 mg/m2 per week) plus cisplatin (100 mg/m2 every 4 weeks) to single-agent cisplatin (100 mg/m2 every 4 weeks) in patients with Stage IV or Stage IIIb NSCLC patients with malignant pleural effusion or multiple lesions in more than one lobe who were not previously treated with chemotherapy. Patients included in the study had a performance status of 0 or 1, and 34% had received prior surgery and/or radiotherapy.
- Characteristics of the 432 randomized patients are provided in TABLE 1. Two hundred and twelve patients received vinorelbine tartrate plus cisplatin and 210 received single-agent cisplatin. The primary objective of this trial was to compare survival between the 2 treatment groups. Survival (FIGURE 1) for patients receiving vinorelbine tartrate plus cisplatin was significantly better compared to the patients who received single-agent cisplatin. The results of this trial are summarized in TABLE 1.
- Vinorelbine Tartrate plus Cisplatin versus Vindesine plus Cisplatin versus Single-Agent Vinorelbine Tartrate
- In a large European clinical trial, 612 patients with Stage III or IV NSCLC, no prior chemotherapy, and WHO Performance Status of 0, 1, or 2 were randomized to treatment with single-agent vinorelbine tartrate (30 mg/m2 per week), vinorelbine tartrate (30 mg/m2 per week) plus cisplatin (120 mg/m2 days 1 and 29, then every 6 weeks), and vindesine (3 mg/m2 per week for 7 weeks, then every other week) plus cisplatin (120 mg/m2 days 1 and 29, then every 6 weeks). Patient characteristics are provided in TABLE 1. Survival was longer in patients treated with vinorelbine tartrate plus cisplatin compared to those treated with vindesine plus cisplatin (FIGURE 2). Study results are summarized in TABLE 1.
Dose-Ranging Study
- A dose-ranging study of vinorelbine tartrate (20, 25, or 30 mg/m2 per week) plus cisplatin (120 mg/m2 days 1 and 29, then every 6 weeks) in 32 patients with NSCLC demonstrated a median survival of 10.2 months. There were no responses at the lowest dose level; the response rate was 33% in the 21 patients treated at the 2 highest dose levels.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Vinorelbine in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Vinorelbine in the drug label.
How Supplied
- Vinorelbine Injection UPS is a clear, colorless to pale yellow solution in water for injection, containing 10 mg vinorelbine tartrate, USP per mL. Vinorelbine Injection USP is available as follows:

Storage
- Store the vials under refrigeration at 2° to 8°C (36° to 46°F) in the carton. * Protect from light. DO NOT FREEZE.
Images
Drug Images
{{#ask: Page Name::Vinorelbine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Vinorelbine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Vinorelbine Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Vinorelbine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- VINORELBINE ®[12]
Look-Alike Drug Names
There is limited information regarding Vinorelbine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Andersson M, Lidbrink E, Bjerre K, Wist E, Enevoldsen K, Jensen AB; et al. (2011). "Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study". J Clin Oncol. 29 (3): 264–71. doi:10.1200/JCO.2010.30.8213. PMID 21149659 PMID: 21149659 Check
|pmid=value (help). - ↑ Morris M, Brader KR, Levenback C, Burke TW, Atkinson EN, Scott WR; et al. (1998). "Phase II study of vinorelbine in advanced and recurrent squamous cell carcinoma of the cervix". J Clin Oncol. 16 (3): 1094–8. PMID 9508195 PMID: 9508195 Check
|pmid=value (help). - ↑ Conroy T (2002). "Activity of vinorelbine in gastrointestinal cancers". Crit Rev Oncol Hematol. 42 (2): 173–8. PMID 12007975 PMID: 12007975 Check
|pmid=value (help). - ↑ Fournier C, Hecquet B, Bouffard P, Vert M, Caty A, Vilain MO; et al. (1991). "Experimental studies and preliminary clinical trial of vinorelbine-loaded polymeric bioresorbable implants for the local treatment of solid tumors". Cancer Res. 51 (19): 5384–91. PMID 1913658 PMID: 1913658 Check
|pmid=value (help). - ↑ Bonfante V, Viviani S, Santoro A, Devizzi L, Di Russo A, Zanini M; et al. (1998). "Ifosfamide and vinorelbine: an active regimen for patients with relapsed or refractory Hodgkin's disease". Br J Haematol. 103 (2): 533–5. PMID 9827930 PMID: 9827930 Check
|pmid=value (help). - ↑ Brambilla L, Boneschi V, Fossati S, Ferrucci S, Finzi AF (1997). "Vinorelbine therapy for Kaposi's sarcoma in a kidney transplant patient". Dermatology. 194 (3): 281–3. PMID 9187850 PMID: 9187850 Check
|pmid=value (help). - ↑ Airoldi M, Bumma C, Bertetto O, Gabriele P, Succo G, Pedani F (1998). "Vinorelbine treatment of recurrent salivary gland carcinomas". Bull Cancer. 85 (10): 892–4. PMID 9835866 PMID: 9835866 Check
|pmid=value (help). - ↑ Balzarotti M, Santoro A, Tondini C, Fornier M, Bonadonna G (1996). "Activity of single agent vinorelbine in pretreated non-Hodgkin's lymphoma". Ann Oncol. 7 (9): 970–2. PMID 9006750 PMID: 9006750 Check
|pmid=value (help). - ↑ Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J; et al. (2004). "Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer". N Engl J Med. 350 (4): 351–60. doi:10.1056/NEJMoa031644. PMID 14736927 PMID: 14736927 Check
|pmid=value (help). - ↑ Bajetta E, Di Leo A, Biganzoli L, Mariani L, Cappuzzo F, Di Bartolomeo M; et al. (1996). "Phase II study of vinorelbine in patients with pretreated advanced ovarian cancer: activity in platinum-resistant disease". J Clin Oncol. 14 (9): 2546–51. PMID 8823334 PMID: 8823334 Check
|pmid=value (help). - ↑ Furuse K, Kubota K, Kawahara M, Takada M, Kimura I, Fujii M; et al. (1996). "Phase II study of vinorelbine in heavily previously treated small cell lung cancer. Japan Lung Cancer Vinorelbine Study Group". Oncology. 53 (2): 169–72. PMID 8604245 PMID: 8604245 Check
|pmid=value (help). - ↑ "VINORELBINE- vinorelbine tartrate injection".