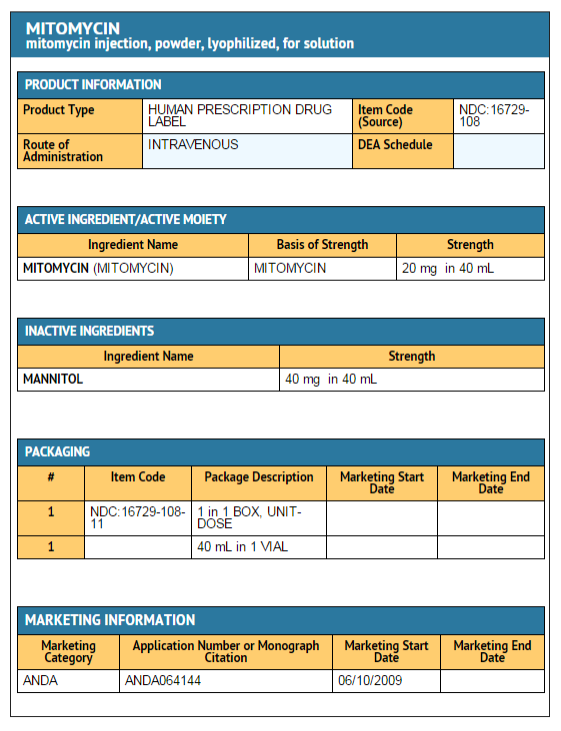
Mitomycin (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Stefano Giannoni [2];Aparna Vuppala, M.B.B.S. [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
Mitomycin should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Appropriate management of therapy and complications is possible only when adequate diagnostic and treatment facilities are readily available.
Bone marrow suppression: Notably thrombocytopenia and leukopenia, which may contribute to overwhelming infections in an already compromised patient, is the most common and severe of the toxic effects of mitomycin. Hemolytic Uremic Syndrome (HUS): A serious complication of chemotherapy, consisting primarily of microangiopathic hemolytic anemia, thrombocytopenia, and irreversible renal failure has been reported in patients receiving systemic mitomycin. The syndrome may occur at any time during systemic therapy with mitomycin as a single agent or in combination with other cytotoxic drugs, however, most cases occur at doses ≥60 mg of mitomycin. Blood product transfusion may exacerbate the symptoms associated with this syndrome. The incidence of the syndrome has not been defined. |
Overview
Mitomycin (injection) is an Antibiotic that is FDA approved for the treatment of disseminated adenocarcinoma of the stomach or pancreas in proven combinations with other approved chemotherapeutic agents and as palliative treatment when other modalities have failed. There is a Black Box Warning for this drug as shown here. Common adverse reactions include loss of appetite, nausea and vomiting and fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Adenocarcinoma of Pancreas and Stomach
- In combination with other chemotherapeutic agents and as palliative treatment when other modalities have failed.
- After full hematological recovery (see guide to dosage adjustment) from any previous chemotherapy, the following dosage schedule may be used at 6 to 8 week intervals:
- 20 mg/m 2 intravenously as a single dose via a functioning intravenous catheter.
- Because of cumulative myelosuppression, patients should be fully reevaluated after each course of mitomycin, and the dose reduced if the patient has experienced any toxicities. Doses greater than 20 mg/m 2 have not been shown to be more effective, and are more toxic than lower doses.
- The following schedule is suggested as a guide to dosage adjustment:
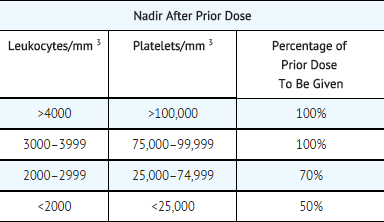
- No repeat dosage should be given until leukocyte count has returned to 4000/mm 3 and a platelet count to 100,000/mm 3.
- When mitomycin is used in combination with other myelosuppressive agents, the doses should be adjusted accordingly.
- If the disease continues to progress after two courses of mitomycin, the drug should be stopped since chances of response are minimal.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mitomycin in adult patients.
Non–Guideline-Supported Use
Carcinoma of Bladder
- 20 mg in 20 mL of water given intravesically weekly for 8 to 12 weeks, followed by monthly instillations for 5 to 6 months.[1]
- Reported effective in the treatment of recurrent multiple superficial bladder tumors
Primary malignant neoplasm of anus
- 10 mg/m(2) IV bolus on days 1 and day 29 [2]
- In combination with fluorouracil and radiation
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Mitomycin (injection) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mitomycin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mitomycin in pediatric patients.
Contraindications
- In patients who have demonstrated a hypersensitive or idiosyncratic reaction to it in the past.
- In patients with thrombocytopenia, coagulation disorder, or an increase in bleeding tendency due to other causes.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
Mitomycin should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Appropriate management of therapy and complications is possible only when adequate diagnostic and treatment facilities are readily available.
Bone marrow suppression: Notably thrombocytopenia and leukopenia, which may contribute to overwhelming infections in an already compromised patient, is the most common and severe of the toxic effects of mitomycin. Hemolytic Uremic Syndrome (HUS): A serious complication of chemotherapy, consisting primarily of microangiopathic hemolytic anemia, thrombocytopenia, and irreversible renal failure has been reported in patients receiving systemic mitomycin. The syndrome may occur at any time during systemic therapy with mitomycin as a single agent or in combination with other cytotoxic drugs, however, most cases occur at doses ≥60 mg of mitomycin. Blood product transfusion may exacerbate the symptoms associated with this syndrome. The incidence of the syndrome has not been defined. |
Patients being treated with mitomycin must be observed carefully and frequently during and after therapy.
- The use of mitomycin results in a high incidence of bone marrow suppression, particularly thrombocytopenia and leukopenia. Therefore, the following studies should be obtained repeatedly during therapy and for at least eight weeks following therapy:
- Platelet count
- White blood cell count, differential
- Hemoglobin
- The occurrence of a platelet count below 100,000/mm 3 or a WBC below 4,000/mm 3 or a progressive decline in either is an indication to withhold further therapy until blood counts have recovered above these levels.
- Patients should be advised of the potential toxicity of this drug, particularly bone marrow suppression.
- Deaths have been reported due to septicemia as a result of leukopenia due to the drug.
- Patients receiving mitomycin should be observed for evidence of renal toxicity.
- Mitomycin should not be given to patients with a serum creatinine greater than 1.7 mg percent.
Adverse Reactions
Clinical Trials Experience
Bone Marrow Toxicity
- This was the most common and most serious toxicity, occurring in 605 of 937 patients (64.4%).
- Thrombocytopenia and/or leukopenia may occur anytime within 8 weeks after onset of therapy with an average time of 4 weeks.
- Recovery after cessation of therapy was within 10 weeks.
- About 25% of the leukopenic or thrombocytopenic episodes did not recover.
- Mitomycin produces cumulative myelosuppression.
Integument and Mucous Membrane Toxicity
- This has occurred in approximately 4% of patients treated with mitomycin.
- Cellulitis at the injection site has been reported and is occasionally severe.
- Stomatitis and alopecia also occur frequently.
- Rashes are rarely reported.
- The most important dermatological problem with this drug, however, is the necrosis and consequent sloughing of tissue which results if the drug is extravasated during injection.
- Extravasation may occur with or without an accompanying stinging or burning sensation and even if there is adequate blood return when the injection needle is aspirated.
- There have been reports of delayed erythema and/or ulceration occurring either at or distant from the injection site, weeks to months after mitomycin, even when no obvious evidence of extravasation was observed during administration.
- Skin grafting has been required in some of the cases.
- Elderly patients may be more susceptible than younger patients to injection site reactions.
Renal Toxicity
- 2% of 1,281 patients demonstrated a statistically significant rise in creatinine.
- There appeared to be no correlation between total dose administered or duration of therapy and the degree of renal impairment.
Pulmonary Toxicity
- This has occurred infrequently but can be severe and may be life-threatening.
- Dyspnea with a nonproductive cough and radiographic evidence of pulmonary infiltrates may be indicative of mitomycin-induced pulmonary toxicity.
- If other etiologies are eliminated, mitomycin therapy should be discontinued.
- Steroids have been employed as treatment of this toxicity, but the therapeutic value has not been determined.
- A few cases of adult respiratory distress syndrome have been reported in patients receiving mitomycin in combination with other chemotherapy and maintained at FlO 2 concentrations greater than 50% perioperatively.
Hemolytic Uremic Syndrome (HUS)
- This serious complication of chemotherapy, consisting primarily of microangiopathic hemolytic anemi (hematocrit ≤25%), thrombocytopenia (≤100,000/mm 3), and irreversible renal failure (serum creatinine ≥1.6 mg/dL) has been reported in patients receiving systemic mitomycin.
- Microangiopathic hemolysis with fragmented red blood cells on peripheral blood smears has occurred in 98% of patients with the syndrome.
- Other less frequent complications of the syndrome may include pulmonary edema (65%), neurologic abnormalities (16%), and hypertension.
- Exacerbation of the symptoms associated with HUS has been reported in some patients receiving blood product transfusions.
- A high mortality rate (52%) has been associated with this syndrome.
- The syndrome may occur at any time during systemic therapy with mitomycin as a single agent or in combination with other cytotoxic drugs.
- Less frequently, HUS has also been reported in patients receiving combinations of cytotoxic drugs not including mitomycin.
- Of 83 patients studied, 72 developed the syndrome at total doses exceeding 60 mg of mitomycin.
- Consequently, patients receiving ≥60 mg of mitomycin should be monitored closely for unexplained anemia with fragmented cells on peripheral blood smear, thrombocytopenia, and decreased renal function.
- The incidence of the syndrome has not been defined.
- Therapy for the syndrome is investigational.
Cardiac Toxicity
- Congestive heart failure, often treated effectively with diuretics and cardiac glycosides, has rarely been reported.
- Almost all patients who experienced this side effect had received prior doxorubicin therapy.
- Acute Side Effects Due to Mitomycin were fever, anorexia, nausea, and vomiting. They occurred in about 14% of 1,281 patients.
Other
- Headache
- Blurring of vision
- Confusion
- Drowsiness
- Syncope
- Fatigue
- Edema
- Thrombophlebitis
- Hematemesis
- Diarrhea
- Pain
- These did not appear to be dose related and were not unequivocally drug related.
- They may have been due to the primary or metastatic disease processes.
- Bladder fibrosis/contraction has been reported with intravesical administration
Postmarketing Experience
Drug Interactions
There is limited information regarding Mitomycin (injection) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Safe use of mitomycin in pregnant women has not been established.
- Teratological changes have been noted in animal studies.
- The effect of mitomycin on fertility is unknown.
Pregnancy Category (AUS): D
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Mitomycin (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Mitomycin (injection) during labor and delivery.
Nursing Mothers
- It is not known if mitomycin is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from mitomycin, it is recommended that nursing be discontinued when receiving mitomycin therapy.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- Insufficient data from clinical studies of mitomycin are available for patients 65 years of age and older to determine whether they respond differently than younger patients.
- Postmarketing surveillance suggests that elderly patients may be more susceptible than younger patients to injection site reactions and hypersensitivity reactions.
- In general, caution should be exercised when prescribing to elderly patients, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Mitomycin (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Mitomycin (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Mitomycin (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Mitomycin (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Mitomycin (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Mitomycin (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
- Patients receiving ≥60 mg of mitomycin should be monitored closely for unexplained anemia with fragmented cells on peripheral blood smear, thrombocytopenia, and decreased renal function.
IV Compatibility
- Unreconstituted mitomycin stored at room temperature is stable for the lot life indicated on the package. Avoid excessive heat (over 40°C, 104°F).
- Reconstituted with Sterile Water for Injection to a concentration of 0.5 mg per mL, mitomycin is stable for 14 days refrigerated or 7 days at room temperature.
- Diluted in various I.V. fluids at room temperature, to a concentration of 20 to 40 micrograms per mL:
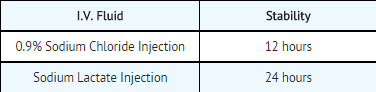
- The combination of mitomycin (5 mg to 15 mg) and heparin (1,000 units to 10,000 units) in 30 mL of 0.9% Sodium Chloride Injection is stable for 48 hours at room temperature.
- Procedures for proper handling and disposal of anticancer drugs should be considered.
- Several guidelines on this subject have been published.
- There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
Overdosage
There is limited information regarding Mitomycin (injection) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Mitomycin (injection) Pharmacology in the drug label.
Mechanism of Action
- Mitomycin (also known as mitomycin and/or mitomycin-C) is an antibiotic isolated from the broth of Streptomyces caespitosus which has been shown to have antitumor activity.
- Mitomycin selectively inhibits the synthesis of deoxyribonucleic acid (DNA).
- The guanine and cytosine content correlates with the degree of mitomycin-induced cross-linking.
- At high concentrations of the drug, cellular RNA and protein synthesis are also suppressed.
Structure
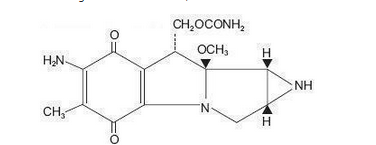
- The compound is heat stable, has a high melting point, and is freely soluble in organic solvents.
- Mitomycin is a blue-violet crystalline powder with the molecular formula of C 15H 18N 4O 5, and a molecular weight of 334.33. Its chemical name is 7-amino-9α-methoxymitosane and it has the following structural formula:
Pharmacodynamics
There is limited information regarding Mitomycin (injection) Pharmacodynamics in the drug label.
Pharmacokinetics
- In humans, mitomycin is rapidly cleared from the serum after intravenous administration.
- Time required to reduce the serum concentration by 50% after a 30 mg bolus injection is 17 minutes.
- After injection of 30 mg, 20 mg, or 10 mg I.V., the maximal serum concentrations were 2.4 mcg/mL, 1.7 mcg/mL, and 0.52 mcg/mL, respectively.
- Clearance is effected primarily by metabolism in the liver, but metabolism occurs in other tissues as well.
- The rate of clearance is inversely proportional to the maximal serum concentration because, it is thought, of saturation of the degradative pathways.
- Approximately 10% of a dose of mitomycin is excreted unchanged in the urine.
- Since metabolic pathways are saturated at relatively low doses, the percent of a dose excreted in urine increases with increasing dose.
- In children, excretion of intravenously administered mitomycin is similar.
Nonclinical Toxicology
There is limited information regarding Mitomycin (injection) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Mitomycin (injection) Clinical Studies in the drug label.
How Supplied
Mitomycin for Injection USP
- NDC 16729-115-05—Each amber vial contains 5 mg mitomycin, individually packed in single carton.
- NDC 16729-108-11—Each amber vial contains 20 mg mitomycin, individually packed in single carton.
- NDC 16729-116-38—Each amber vial contains 40 mg mitomycin, individually packed in single carton.
Storage
- Store dry powder at 25°C, excursion permitted between 15°C and 30°C, protected from light.
- Avoid excessive heat, over 40 °C (104° F).
- Protect reconstituted solution from light.
- Store solution under refrigeration 2° to 8 °C (36° to 46°F), discard after 14 days.
- If unrefrigerated, discard after 7 days.
Images
Drug Images
{{#ask: Page Name::Mitomycin (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

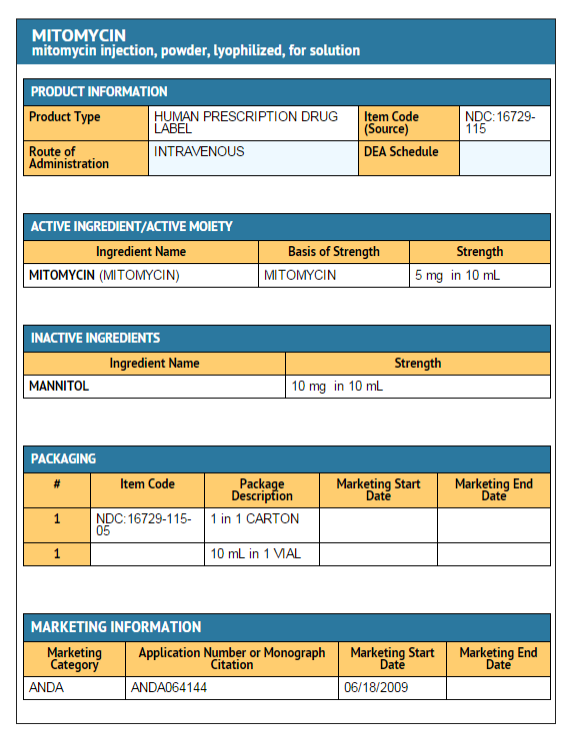
{{#ask: Label Page::Mitomycin (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Mitomycin (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Mitomycin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Mutamycin[3]
Look-Alike Drug Names
There is limited information regarding Mitomycin (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Hetherington JW, Newling DW, Robinson MR, Smith PH, Adib RS, Whelan P (1987). "Intravesical mitomycin C for the treatment of recurrent superficial bladder tumours". Br J Urol. 59 (3): 239–41. PMID 3105631.
- ↑ Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, Thomas CR; et al. (2008). "Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial". JAMA. 299 (16): 1914–21. doi:10.1001/jama.299.16.1914. PMID 18430910.
- ↑ "MITOMYCIN- mitomycin injection, powder, lyophilized, for solution".
{{#subobject:
|Label Page=Mitomycin (injection) |Label Name=Mitomycin Package.png
}}
{{#subobject:
|Label Page=Mitomycin (injection) |Label Name=Mitomycin Package 2.png
}}
{{#subobject:
|Label Page=Mitomycin (injection) |Label Name=Mitomycin Package 3.png
}}