Mipomersen
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: RISK OF HEPATOTOXICITY
See full prescribing information for complete Boxed Warning.
* Kynamro can cause elevations in transaminases. In the Kynamro clinical trial in patients with HoFH, 4 (12%) of the 34 patients treated with Kynamro compared with 0% of the 17 patients treated with placebo had at least one elevation in alanine aminotransferase (ALT) ≥ 3x upper limit of normal (ULN). There were no concomitant clinically meaningful elevations of total bilirubin, international normalized ratio (INR) or partial thromboplastin time (PTT).
|
Overview
Mipomersen is a lipid-lowering medication that is FDA approved for the {{{indicationType}}} of hypercholesterolemia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include injection site reactions, flu-like symptoms, nausea, headache, and elevations in serum transaminases, specifically ALT.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Homozygous Familial Hypercholesterolemia (HoFH)
- KynamroTM is indicated as an adjunct to lipid-lowering medications and diet to reduce low density lipoprotein-cholesterol (LDL-C), apolipoprotein B (apo B), total cholesterol (TC), and non-high density lipoprotein-cholesterol (non-HDL-C) in patients with homozygous familial hypercholesterolemia (HoFH).
- Limitations of Use
- The safety and effectiveness of Kynamro have not been established in patients with hypercholesterolemia who do not have HoFH.
- The effect of Kynamro on cardiovascular morbidity and mortality has not been determined.
- The safety and effectiveness of Kynamro as an adjunct to LDL apheresis have not been established; therefore, the use of Kynamro as an adjunct to LDL apheresis is not recommended.
- Dosing Information
- Before beginning treatment with Kynamro, measure transaminases (ALT, AST), alkaline phosphatase, and total bilirubin.
- The recommended dose of Kynamro is 200 milligrams (mg) once weekly as a subcutaneous injection.
- Kynamro is intended for subcutaneous use only. Do not administer intramuscularly or intravenously.
- The injection should be given on the same day every week, but if a dose is missed, the injection should be given at least 3 days from the next weekly dose.
- After initiation of Kynamro therapy lipid levels should be monitored at least every 3 months for the first year. Maximal reduction of LDL-C may be seen with Kynamro therapy after approximately 6 months (based on the time to steady state seen in clinical studies). Health care providers should assess the patient’s LDL-C level after 6 months to determine if the LDL-C reduction achieved with Kynamro is sufficiently robust to warrant the potential risk of liver toxicity.
- Administration
- Each vial or pre-filled syringe of Kynamro provides 200 mg of mipomersen sodium in a deliverable volume of 1 milliliter (mL) of solution and is intended for single-use only.
- The Kynamro vial or pre-filled syringe should be removed from 2-8°C (36-46°F) refrigerated storage and allowed to reach room temperature for at least 30 minutes prior to administration.
- Parenteral drug products should be inspected visually prior to administration. If the solution is cloudy or contains visible particulate matter, the contents must not be injected and the product should be returned to the pharmacy.
- The first injection administered by the patient or caregiver should be performed under the guidance and supervision of an appropriately qualified health care professional.
- Kynamro should be injected into the abdomen, thigh region, or outer area of the upper arm. Kynamro should not be injected in areas of active skin disease or injury such as sunburns, skin rashes, inflammation, skin infections, active areas of psoriasis, etc. Areas of tattooed skin and scarring should also be avoided.
- Adjustments for Patients Developing transaminase Elevations
- Table 1 summarizes recommendations for monitoring for patients who develop elevated transaminases during therapy with Kynamro.
- If transaminase elevations are accompanied by clinical symptoms of liver injury (e.g., nausea, vomiting, abdominal pain, fever, jaundice, lethargy, flu-like symptoms), increases in bilirubin ≥ 2x ULN, or active liver disease, discontinue treatment with Kynamro and investigate to identify the probable cause.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mipomersen in adult patients.
Non–Guideline-Supported Use
Hypercholesterolemia
- Dosing Information
- Mipomersen 200 mg subQ once weekly.[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness have not been established in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mipomersen in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mipomersen in pediatric patients.
Contraindications
- Moderate or severe hepatic impairment (Child-Pugh B or C) or active liver disease, including unexplained persistent elevations of serum transaminases.
- Patients with a known hypersensitivity to any component of this product.
Warnings
|
WARNING: RISK OF HEPATOTOXICITY
See full prescribing information for complete Boxed Warning.
* Kynamro can cause elevations in transaminases. In the Kynamro clinical trial in patients with HoFH, 4 (12%) of the 34 patients treated with Kynamro compared with 0% of the 17 patients treated with placebo had at least one elevation in alanine aminotransferase (ALT) ≥ 3x upper limit of normal (ULN). There were no concomitant clinically meaningful elevations of total bilirubin, international normalized ratio (INR) or partial thromboplastin time (PTT).
|
Risk of Hepatotoxicity
- Kynamro can cause elevations in transaminases and hepatic steatosis, as described below. To what extent Kynamro-associated hepatic steatosis promotes the elevations in transaminases is unknown. There is concern that Kynamro could induce steatohepatitis, which can progress to cirrhosis over several years. The clinical studies supporting the safety and efficacy of Kynamro in HoFH would have been unlikely to detect this adverse outcome given their size and duration.
Elevation of Transaminases
- Kynamro can cause increases in serum transaminases (alanine aminotransferase [ALT] and/or aspartate aminotransferase [AST]). In the clinical trial, 4 (12%) of the 34 subjects with HoFH treated with Kynamro compared to 0% of the 17 subjects treated with placebo had an elevation in ALT ≥ 3x ULN, and 3 (9%) of those treated with Kynamro compared to 0% treated with placebo had at least one elevation in ALT ≥ 5x ULN.
- Measure a full liver panel to include ALT, AST, total bilirubin, and alkaline phosphatase before initiation of treatment with Kynamro. Kynamro is contraindicated in patients with moderate or severe hepatic impairment, or active liver disease, including unexplained persistent elevations of serum transaminases. If the baseline liver-related tests are abnormal, consider initiating Kynamro after an appropriate work-up and the baseline abnormalities are explained or resolved. During the first year, conduct liver-related tests monthly (ALT and AST, at a minimum). After the first year, conduct these tests at least every 3 months. Discontinue Kynamro for persistent or clinically significant elevations.
- If transaminase elevations are accompanied by clinical symptoms of liver injury (e.g., nausea, vomiting, abdominal pain, fever, jaundice, lethargy, flu-like symptoms), increases in bilirubin ≥ 2x ULN, or active liver disease, discontinue treatment with Kynamro and identify the probable cause.
Hepatic Steatosis
- Kynamro increases hepatic fat (steatosis) with or without concomitant increases in transaminases. Hepatic steatosis is a risk factor for advanced liver disease, including steatohepatitis and cirrhosis. The long-term consequences of hepatic steatosis associated with Kynamro therapy are unknown. During the clinical trials in patients with heterozygous familial hypercholesterolemia (HeFH) and hyperlipidemia, the median absolute increase in hepatic fat was 10% after 26 weeks of treatment, from 0% at baseline, measured by magnetic resonance imaging (MRI).
- Alcohol may increase levels of hepatic fat and induce or exacerbate liver injury. It is recommended that patients taking Kynamro should consume no more than one alcoholic drink per day.
- Caution should be exercised when Kynamro is used with other medications known to have potential for hepatotoxicity, for example isotretinoin, amiodarone, acetaminophen (>4 g/day for ≥ 3 days/week), methotrexate, tetracyclines, and tamoxifen. The effect of concomitant administration of Kynamro with other hepatotoxic medications is unknown. More frequent monitoring of liver-related tests may be warranted.
- Mipomersen has not been studied concomitantly with other LDL-lowering agents that can also increase hepatic fat. Therefore, the combined use of such agents is not recommended.
Kynamro REMS
- Because of the risk of hepatotoxicity, Kynamro is available only through a limited program under the REMS. Under the Kynamro REMS, only certified healthcare providers and pharmacies may prescribe and distribute Kynamro. Further information is available at www.KynamroREMS.com or by telephone at 1-877-Kynamro (1-877-596-2676).
Injection Site Reactions
- Injection site reactions have been reported in 84% of patients receiving Kynamro therapy. These local reactions typically consist of one or more of the following: erythema, pain, tenderness, pruritus and local swelling. Injection site reactions do not occur with all injections but resulted in discontinuation of therapy in 5% of patients in pooled Phase 3 trials. To minimize the potential for injection site reactions, proper technique for subcutaneous administration should be followed.
Flu-Like Symptoms
- Flu-like symptoms have been reported in 30% of patients receiving Kynamro therapy and include one or more of the following: influenza-like illness, pyrexia, chills, myalgia, arthralgia, malaise or fatigue. Flu-like symptoms, which typically occur within 2 days after an injection, do not occur with all injections but resulted in discontinuation of therapy in 3% of patients in pooled Phase 3 trials.
Adverse Reactions
Clinical Trials Experience
Clinical Trials
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trial of another drug and may not reflect the rates observed in patients in clinical practice.
- Safety data are based on pooled results from four Phase 3, randomized, double-blind, placebo-controlled trials with a total of 390 patients of which 261 patients received weekly subcutaneous injections of 200 mg of Kynamro and 129 patients received placebo for a median treatment duration of 25 weeks (age range 12-81 years, 47% women, 84% Caucasian, 10% Blacks, 3% Asian, 3% other). For the 141 participants who subsequently enrolled in the open-label extension trial, the mean length of study treatment, including exposure to Kynamro in the index study, was 19.8 months and the median was 18.2 months. A total of 41 individuals with HoFH were exposed to Kynamro for at least 6 months and 25 were exposed for at least 12 months.
- Eighteen percent of patients on Kynamro and 2% of patients on placebo discontinued treatment due to adverse reactions. The five most common adverse reactions in patients treated with Kynamro that led to treatment discontinuation and occurred at a rate greater than placebo were: injection site reactions (5.0%), alanine aminotransferase increased (3.4%), flu-like symptoms (2.7%), aspartate aminotransferase increased (2.3%), and liver function test abnormal (1.5%).
Common Adverse Reactions
- Table 2 enumerates adverse reactions that occurred among pooled Phase 3 patients treated with Kynamro at an incidence that was at least 2% more than that observed in the placebo-treated patients, listed by system organ class and frequency (MedDRA v.13.0). Similar types and severities of adverse reactions were observed across all populations in this pooled table including the subset of patients with HoFH.
- In the pooled Phase 3 trials, neoplasms (benign and malignant) were reported in 4% of patients receiving Kynamro and 0% of patients receiving placebo. In addition, 9% of patients receiving Kynamro and 3% of patients receiving placebo developed 1+ or greater proteinuria by dipstick measurement by the end of the trial.
- In the open-label extension trial, one case of hypersensitivity reaction with angioedema and one case of glomerular nephritis were reported.
Transaminase Elevations
- In the pooled, placebo-controlled clinical trials with Kynamro, elevated serum transaminase levels, mainly ALT, have been observed as presented in Table 3. Elevated ALT levels ≥ 3x ULN have been reported on two consecutive occasions at least 7 days apart in 8.4% of patients receiving Kynamro therapy (versus 0% of placebo patients) with 16.5% of patients receiving Kynamro therapy having at least 1 result that was ≥ 3x ULN (versus 0.8% for placebo patients). The ALT elevations observed in the pooled, placebo-controlled trials were generally accompanied by lesser AST elevations and were not associated with increased total bilirubin, changes in INR or PTT, nor by decreased albumin levels. After stopping therapy, in the patients in whom an elevation was observed, transaminase elevations trended toward baseline over a period of weeks to months.
Hepatic Steatosis
- Increases in liver fat as measured by MRI were greater in patients receiving Kynamro therapy than in patients receiving placebo. Data from Phase 3 supportive trials in patients with heterozygous familial hypercholesterolemia and coronary artery disease and in patients with high risk hypercholesterolemia demonstrated after 26 weeks of treatment, a median nominal increase in fat fraction of 9.6% relative to baseline following Kynamro therapy versus a nominal 0.02% change in the placebo group (mean increases were 12.2% mipomersen vs 0.4% placebo). The maximum change in fat fraction was 46% for the Kynamro group and 28% for the placebo group. Sixty-two percent of patients receiving Kynamro developed a 5% or greater increase in hepatic fat versus 8% of patients receiving placebo. In general, these elevations in fat fraction decreased when assessed by MRI performed 24 weeks after cessation of Kynamro in the Phase 3 trial of patients with high-risk hypercholesterolemia. In the open-label extension trial, among individuals with a measurement at baseline and at 12 months or longer on Kynamro, 25% had an average liver fat fraction > 20% on at least one occasion.
Injection Site Reactions
- The most commonly-reported adverse reactions were injection site reactions occurring in 84% of patients receiving Kynamro versus 33% of placebo treated patients. The most common injection site reactions were erythema (59%), pain (56%), hematoma (32%), pruritus (29%), swelling (18%) and discoloration (17%). Injection site reactions did not occur with every injection. Injection site reactions resulted in discontinuation of Kynamro in 5% of patients. Recall reactions, consisting of local erythema, tenderness and/or pruritus at previous injection sites when subsequent injections were administered, were observed in 8% of patients, all of whom were receiving Kynamro.
Flu-like Symptoms
- Flu-like symptoms, defined as any one of the following: influenza-like illness, pyrexia, chills, myalgia, arthralgia, malaise or fatigue and occurring within 2 days of injection, have been reported more frequently in patients receiving Kynamro (29.9%) versus placebo (16.3%) in the pooled Phase 3 studies. Flu-like symptoms did not occur with all injections. Flu-like symptoms resulted in discontinuation of Kynamro in 2.7% of patients. In the open-label extension trial, in which all patients received Kynamro therapy, 66% reported flu-like symptoms, 25% discontinued treatment due to flu-like symptoms and 9% experienced severe flu-like symptoms.
Immunogenicity
- In the pooled Phase 3 trials, 38% of Kynamro-treated patients tested positive for anti-Kynamro antibodies during the 6-month trials. Efficacy results in the Phase 3 trials in patients who tested positive for anti-Kynamro antibodies were similar to patients who remained negative for antibodies (mean LDL-C percent change from baseline was -32% for antibody-positive and -34% for antibody-negative participants). In the open-label extension trial, approximately 72% of patients receiving Kynamro therapy tested positive for anti-Kynamro antibodies (35% with titers >3200). The incidence of flu-like symptoms and the incidence of discontinuation of Kynamro were higher in antibody-positive patients. Antibodies to Kynamro were associated with higher trough levels for the drug. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Kynamro with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Mipomersen in the drug label.
Drug Interactions
- No clinically relevant pharmacokinetic interactions were reported between Kynamro and warfarin, or between Kynamro and simvastatin or ezetimibe. Additionally, coadministration of Kynamro with warfarin did not result in a pharmacodynamic interaction as determined by INR, aPTT and PT.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- There are no adequate and well-controlled studies in pregnant women. Reproduction and embryofetal development studies performed in mice at doses up to 87.5 mg/kg/wk given by subcutaneous administration from mating through organogenesis and in pregnant rabbits given 52.5 mg/kg/wk, show no evidence of impaired fertility or harm to the fetus at 2 (mice) to 5 (rabbits) times clinical exposure at a 200 mg/wk therapeutic dose. Because animal reproduction studies are not always predictive of the human response, this drug should be used during pregnancy only if clearly needed.
- Pregnant rats given subcutaneous doses of 7, 35, 70 mg/kg/wk mipomersen sodium from gestation day 6 through weaning on lactation day 20, resulted in decreased rat pup survival at 70 mg/kg/wk, 3-times clinical exposure at a 200 mg/wk therapeutic dose based on body surface area comparisons across species. Dose related decreases in pup body weights, impaired reflexes and grip strength were observed at 35 mg/kg/wk (2-times the anticipated human dose). Levels of mipomersen in rat milk were very low (≤ 0.92 µg/mL at subcutaneous doses up to 70 mg/kg/wk). Due to the poor oral bioavailability of mipomersen sodium, it was considered unlikely that these low milk exposure levels adversely affected the pups during lactation.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Mipomersen in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Mipomersen during labor and delivery.
Nursing Mothers
- It is not known whether Kynamro is excreted in human milk. Because many drugs are excreted in human milk a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
- Levels of mipomersen present in rat milk were low (≤ 0.92 µg/mL) given subcutaneous doses up to 70 mg/kg/wk. Oral bioavailability is expected to be less than 10%. However a risk to newborns/infants cannot be excluded, therefore caution should be used when Kynamro is administered to a nursing woman.
- Lactating rats administered mipomersen sodium at doses up to 70 mg/kg/wk (3-times the anticipated systemic exposure from a 200 mg/wk dose, based on body surface area comparison) consumed less food while nursing. This correlated with reduced weight gain in the rat pups, and decreased pup survival in litters of dams given 70 mg/kg/wk.
Pediatric Use
- Safety and effectiveness have not been established in pediatric patients.
- A juvenile toxicity study was conducted in rats at doses up to 50 mg/kg/wk (2-times the systemic exposure from a 200 mg/wk clinical dose based on body surface area comparisons). Doses > 10 mg/kg/wk were associated with reduced body weight gain in young rats, but had no effect on long bone growth or sexual development.
Geriatic Use
- Clinical studies of Kynamro did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Of the 51 patients enrolled in the Phase 3 trial in HoFH, the mean age was 31 years and the oldest patient in the trial was 53 years. Of the 261 patients who received Kynamro in the pooled Phase 3 trials, 59 (22.6%) were ≥ 65 years old and 10 (3.8%) were ≥ 75 years old. In the pooled Phase 3 trials, patients ≥ 65 years of age treated with Kynamro had a higher incidence of hypertension and peripheral edema compared to placebo patients in this age group, as well as compared to the younger Kynamro-treated age group. Hepatic steatosis was also reported with greater frequency in the ≥ 65 group (13.6%) compared to the <65 group (10.4%).
Gender
There is no FDA guidance on the use of Mipomersen with respect to specific gender populations.
Race
There is no FDA guidance on the use of Mipomersen with respect to specific racial populations.
Renal Impairment
- The safety and efficacy of Kynamro treatment in patients with known renal impairment or in patients undergoing renal dialysis have not been established. Due to the lack of clinical data and Kynamro’s renal safety profile, Kynamro is not recommended in patients with severe renal impairment, clinically significant proteinuria, or on renal dialysis.
Hepatic Impairment
- The safety and efficacy of Kynamro treatment in patients with known hepatic impairment have not been established. Kynamro is contraindicated in patients with clinically significant hepatic dysfunction, which may include persistent elevations of transaminases.
Females of Reproductive Potential and Males
- Kynamro may cause fetal harm. Females who become pregnant during Kynamro therapy should notify their healthcare provider.
- Contraception
- Females of reproductive potential should use effective contraception during Kynamro therapy.
Immunocompromised Patients
There is no FDA guidance one the use of Mipomersen in patients who are immunocompromised.
Administration and Monitoring
Administration
- Subcutaneous
Monitoring
There is limited information regarding Monitoring of Mipomersen in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Mipomersen in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- There have been no reports of overdose with Kynamro treatment. In clinical trials, patients receiving higher doses of Kynamro (300 mg and 400 mg once weekly for 13 weeks) experienced adverse reactions similar to the adverse reactions experienced by patients receiving treatment with 200 mg once weekly but at slightly higher rates and greater severity. Liver-related tests should be monitored.
Management
- Although there is no information on the effect of hemodialysis in treating an overdose with mipomersen, hemodialysis is unlikely to be useful in overdose management since mipomersen is highly bound to plasma proteins.
Chronic Overdose
There is limited information regarding Chronic Overdose of Mipomersen in the drug label.
Pharmacology
Mipomersen
| |
| Systematic (IUPAC) name | |
| 2'-O-(2-methoxyethyl)-P-thioguanylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiocytidylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiocytidylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiouridylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiocytidylyl-(3'→5')-2'-deoxy-P-thioadenylyl-(3'→5')-2'-deoxy-Pthioguanylyl-(3'→5')P-thiothymidylyl-(3'→5')-2'-deoxy-5-methyl-P-thiocytidylyl-(3'→5')-P-thiothymidylyl-(3'→5')-2'-deoxy-P-thioguanylyl-(3'→5')-2'-deoxy-5-methyl-P-thiocytidylyl-(3'→5')-P-thiothymidylyl-(3'→5')-P-thiothymidylyl-(3'→5')-2'-deoxy-5-methyl-P-thiocytidylyl-(3'→5')-2'-O-(2-methoxyethyl)-Pthioguanylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiocytidylyl-(3'→5')-2'-O-(2-methoxyethyl)-P-thioadenylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiocytidylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methylcytidine nonadecasodiumsalt | |
| Identifiers | |
| CAS number | |
| ATC code | C10 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 7594.80 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | Injection |
Mechanism of Action
- Mipomersen is an antisense oligonucleotide targeted to human messenger ribonucleic acid (mRNA) for apo B-100, the principal apolipoprotein of LDL and its metabolic precursor, VLDL. Mipomersen is complementary to the coding region of the mRNA for apo B-100, and binds by Watson and Crick base pairing. The hybridization of mipomersen to the cognate mRNA results in RNase H-mediated degradation of the cognate mRNA thus inhibiting translation of the apo B-100 protein.
- The in vitro pharmacologic activity of mipomersen was characterized in human hepatoma cell lines (HepG2, Hep3B) and in human and cynomolgus monkey primary hepatocytes. In these experiments, mipomersen selectively reduced apo B mRNA, protein and secreted protein in a concentration- and time-dependent manner. The effects of mipomersen were shown to be highly sequence-specific. The binding site for mipomersen lies within the coding region of the apo B mRNA at the position 3249-3268 relative to the published sequence GenBank accession number NM_000384.1.
Structure
- Kynamro (mipomersen sodium) Injection is a sterile, preservative-free, clear, colorless to slightly yellow, aqueous solution for subcutaneous injection. Kynamro is supplied in single-use, 2 mL, clear glass vials or single-use, 1 mL, clear glass pre-filled syringes filled to deliver 1 mL of solution containing 200 mg of mipomersen sodium (200 mg per 1 mL). Kynamro is formulated in water for injection and may include hydrochloric acid and/or sodium hydroxide for pH adjustment to 7.5 – 8.5.
- Mipomersen sodium is an oligonucleotide inhibitor of apo B-100 synthesis. ApoB is the principal apolipoprotein of LDL and its metabolic precursor, very low density lipoprotein (VLDL). Mipomersen inhibits synthesis of apoB by sequence-specific binding to its messenger ribonucleic acid (mRNA) resulting in degradation of the mRNA through enzyme-mediated pathways or disruption of mRNA function through binding alone.
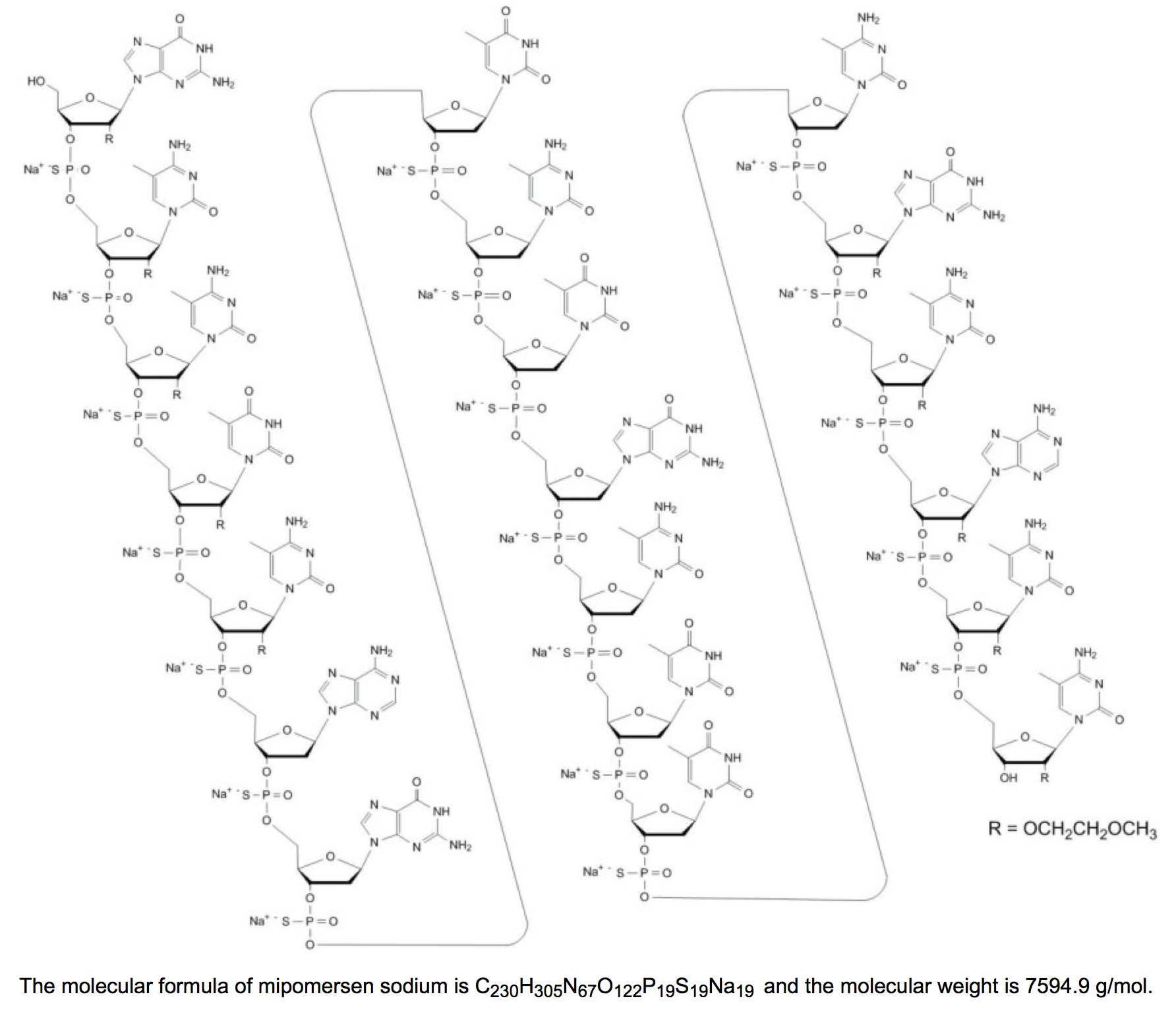
- Mipomersen sodium is a synthetic phosphorothioate oligonucleotide sodium salt, 20 nucleotides in length, with the following sequence:
- 5'-GMeCMeCMeUMeC AGTMeCTGMeCTTMeC GMeCAMeCMeC- 3'
- where the underlined residues are 2'-O-(2-methoxyethyl) nucleosides; all other residues are 2'-deoxynucleosides. Substitution at the 5-position of the cytosine (C) and uracil (U) bases with a methyl group is indicated by Me.
- Mipomersen sodium is represented by the following structural formula:
Pharmacodynamics
- At a concentration of 3.8 times the Cmax of the maximum recommended dose (200 mg subcutaneous injection), mipomersen does not prolong the QTc interval to any clinically relevant extent.
Pharmacokinetics
- Single- and multiple-dose pharmacokinetics of mipomersen in healthy volunteers and in patients with FH and non-FH has shown that mipomersen plasma exposure increases with increasing dose in the range of 30 mg to 400 mg.
Absorption
- Following subcutaneous injection, peak concentrations of mipomersen are typically reached in 3 to 4 hours. The estimated plasma bioavailability of mipomersen following subcutaneous administration over a dose range of 50 mg to 400 mg, relative to intravenous administration, ranged from 54% to 78%.
Distribution
- Mipomersen is highly bound to human plasma proteins (≥ 90%) at clinically relevant concentrations (1-8 µg/mL). Mipomersen has a distribution plasma half-life of approximately 2 to 5 hours.
- With once weekly dosing, plasma trough levels increase over time and approach steady-state, typically within 6 months.
Metabolism
- Mipomersen is not a substrate for CYP450 metabolism, and is metabolized in tissues by endonucleases to form shorter oligonucleotides that are then substrates for additional metabolism by exonucleases.
Excretion
- The elimination of mipomersen involves both metabolism in tissues and excretion, primarily in urine. Both mipomersen and putative shorter oligonucleotide metabolites were identified in human urine. Urinary recovery was limited in humans with less than 4% within the 24 hours post dose. Following subcutaneous administration, elimination half-life for mipomersen is approximately 1 to 2 months.
Drug Interactions
- No clinically relevant pharmacokinetic interactions were reported between mipomersen and warfarin, or between mipomersen and simvastatin or ezetimibe. The results of these studies are summarized in Figures 1 and 2.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In a subcutaneous carcinogenicity study in mice, mipomersen sodium was administered for up to 104 weeks at doses of 5, 20, 60 mg/kg/week. There were statistically significant increases in the incidences of hepatocellular adenoma and combined adenoma and carcinoma in female mice at 60 mg/kg/wk (2-times the systemic clinical exposure at 200 mg/wk, based on a body surface area comparison) for both mipomersen sodium and the mouse-specific analog. This dose also resulted in statistically significant increases in the incidence of hemangiosarcomas in female mice and fibrosarcomas of the skin/subcutis in male mice.
- In a subcutaneous carcinogenicity study in rats, mipomersen sodium was administered for up to 104 weeks at doses of 3, 10, 20 mg/kg/wk. The incidence of fibrosarcomas of the skin/subcutis and the combination of fibroma, fibrosarcomas and malignant fibrous histiocytoma of the skin/subcutis was statistically significantly increased in female rats at 10 mg/kg/wk, at less than clinical exposure at the 200 mg/wk dose based on body surface area comparisons. Both sexes of rats also had statistically significant increases in the incidence of malignant fibrous histiocytoma of the skin/subcutis at 20 mg/kg/wk (at clinical exposure at the 200 mg/wk dose based on body surface area comparisons.
- Mipomersen did not exhibit genotoxic potential in a battery of studies, including the in vitro Bacterial Reverse Mutation (Ames) assay, an in vitro cytogenetics assay using a mouse lymphoma cell line, and an in vivo micronucleus assay in mice.
- Mipomersen sodium had no effect on fertility in mice at doses up to 87.5 mg/kg/wk (2-times clinical exposure at the 200 mg/wk dose based on body surface area comparisons).
Animal Pharmacology and/or Toxicology
- The principal target organs for mipomersen pathology are the kidneys and liver. These organs represent the highest distribution of compound, and exhibit microscopic changes reflective of cellular uptake in macrophages. The most widespread toxicological effect of mipomersen was a spectrum of inflammatory changes in numerous organs, including lymphohistiocytic cell infiltrates and increases in lymphoid organ weights, associated with increases in plasma cytokines, chemokines and total serum IgG. In a chronic monkey study, multi-focal intimal hyperplasia with mixed inflammatory infiltrates was evident in vascular beds in 2 of 6 monkeys treated for 12 months with 30 mg/kg/week with a no-observed-adverse-effect-level (NOAEL) of 10 mg/kg/week (approximately equal to clinical exposures anticipated from a 200 mg/wk dose based on body surface area comparisons across species).
Clinical Studies
- The safety and effectiveness of Kynamro, given as 200 mg weekly subcutaneous injections, as an adjunct to lipid-lowering medications in individuals with HoFH were evaluated in a multinational, randomized (34 Kynamro; 17 placebo), placebo-controlled, 26-week trial in 51 patients with HoFH. A diagnosis of functional HoFH was defined by the presence of at least one of the following clinical or laboratory criteria: (1) history of genetic testing confirming 2 mutated alleles at the LDLr gene locus, or (2) documented history of untreated LDL-C > 500 mg/dL and at least one of the criteria (a) tendinous and/or cutaneous xanthoma prior to age 10 years or (b) documentation of elevated LDL-C > 190 mg/dL prior to lipid-lowering therapy consistent with HeFH in both parents. In case a parent was not available, a history of coronary artery disease in a first degree male relative of the parent younger than 55 years or first degree female relative of the parent younger than 60 years was acceptable.
- The baseline demographic characteristics were well-matched between the Kynamro and placebo patients. The mean age was 32 years (range, 12 to 53 years), the mean body mass index (BMI) was 26 kg/m2, 43% were men, and the majority (75%) were Caucasian. In 50 of 51 (98%) patients, the background therapy of maximally tolerated lipid-lowering medication included statins. In total, 44 of the 50 (88%) patients were on maximum-dose statin therapy with or without other lipid-lowering medications. Thirty-eight of the 50 (76%) patients were also taking at least one other lipid-lowering medication, most commonly ezetimibe in 37 of 50 (74%) patients; patients were not on LDL apheresis. Eighty-two percent of the Kynamro group and 100% of the placebo group completed the efficacy endpoint at week 28. Adverse events contributed to premature discontinuation for four patients, all in the Kynamro group [see Adverse Reactions (6)].
- The primary efficacy endpoint was percent change in LDL-C from baseline to Week 28. At Week 28, the mean and median percent changes in LDL-C from baseline were -25% (p<0.001) and -19%, respectively, for the Kynamro group. The mean and median treatment difference from placebo was -21% (95% confidence interval [CI]: -33, -10) and -19%, respectively. Changes in lipids and lipoproteins through the efficacy endpoint at Week 28 are presented in Table 4.
- LDL-C percent changes from baseline with Kynamro were variable among individuals with HoFH ranging from a 2% increase to an 82% reduction. The LDL-C percent changes from baseline in the placebo group range from a 43% increase to a 33% reduction. Mean LDL-C percent changes over time are presented in Figure 3.
How Supplied
- Kynamro is supplied in single-use, 2 mL, clear glass vials or single-use, 1 mL, clear pre-filled syringes with staked needles. Each single-use vial or single-use pre-filled syringe of Kynamro is filled to deliver 1 mL of 200 mg/mL solution containing 200 mg of mipomersen sodium.
- Kynamro is available in cartons containing 1 or 4 vials and 1 or 4 pre-filled syringes.
- Pack of 1 vial: NDC 58468-0190-1
- Pack of 4 vials: NDC 58468-0190-2
- Pack of 1 pre-filled syringe: NDC 58468-0191-1
- Pack of 4 pre-filled syringe: NDC 58468-0191-2
- Store refrigerated Kynamro at 2-8 °C (36-46 °F). Kynamro should be protected from light and kept in the original carton until time of use. When refrigeration is not available Kynamro may be stored at or below 30 °C (86 °F), away from heat sources, for up to 14 days. Do not use Kynamro after the expiration date on the label. This product contains no preservatives; any unused drug remaining in vial after extracting 1 mL for injection must be safely discarded.
Storage
There is limited information regarding Mipomersen Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Mipomersen |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Mipomersen |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information



Precautions with Alcohol
- Alcohol-Mipomersen interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Kynamro®[2]
Look-Alike Drug Names
- N/A[3]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Thomas, Gregory S. (2013-12-10). "Mipomersen, an apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: a randomized, double-blind, placebo-controlled trial". Journal of the American College of Cardiology. 62 (23): 2178–2184. doi:10.1016/j.jacc.2013.07.081. ISSN 1558-3597. PMID 24013058. Unknown parameter
|coauthors=ignored (help) - ↑ "Kynamro (mipomersen sodium) injection, solution".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Mipomersen |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Mipomersen |Label Name=Mipomersen11.png
}}
{{#subobject:
|Label Page=Mipomersen |Label Name=Mipomersen12.png
}}
{{#subobject:
|Label Page=Mipomersen |Label Name=Mipomersen13.png
}}
{{#subobject:
|Label Page=Mipomersen |Label Name=Mipomersen14.png
}}
{{#subobject:
|Label Page=Mipomersen |Label Name=Mipomersen15.png
}}