Magnesium oxide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Magnesium oxide is a antacid that is FDA approved for the treatment of acid indigestion, upset stomach. Common adverse reactions include diarrhea, allergic reaction.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Relieves: acid indigestion, upset stomach
Directions
- Antacid Directions: (take 1 tablet twice a day or as directed by a physician
- Magnesium Supplement Directions: (take 1 to 2 tablets daily or as directed by a physician
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Magnesium oxide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Magnesium oxide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and efficacy in pediatrics patients has not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Magnesium oxide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Magnesium oxide in pediatric patients.
Contraindications
There is limited information regarding Magnesium oxide Contraindications in the drug label.
Warnings
- Do Not Use
- Do not take more than 2 tablets in a 24 hour period or use the maximum dosage of this product for more than two weeks, except under the advice and supervision of a physician. May have a laxative effect.
- Ask a Doctor if you have:
- Kidney disease
- Ask a Doctor/Pharmacist before use if you are:
- Taking a prescription drug. Antacids may interact with certain prescription drugs.
- Pregnancy or Breast Feeding
- If pregnant or breast feeding, ask a health professional before use.
- Keep Out of Reach of Children
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Magnesium oxide in the drug label.
Postmarketing Experience
Laxative adverse reaction
Drug Interactions
There is limited information regarding Magnesium oxide Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- If pregnant or breast feeding, ask a health professional before use.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Magnesium oxide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Magnesium oxide during labor and delivery.
Nursing Mothers
If pregnant or breast feeding, ask a health professional before use.
Pediatric Use
There is no FDA guidance on the use of Magnesium oxide with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Magnesium oxide with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Magnesium oxide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Magnesium oxide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Magnesium oxide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Magnesium oxide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Magnesium oxide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Magnesium oxide in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Magnesium oxide in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Magnesium oxide in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Magnesium oxide in the drug label.
Pharmacology
Template:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox ECNumberTemplate:Chembox E numberTemplate:Chembox RTECSTemplate:Chembox AppearanceTemplate:Chembox OdourTemplate:Chembox DensityTemplate:Chembox MeltingPtTemplate:Chembox BoilingPtTemplate:Chembox SolubilityInWaterTemplate:Chembox SolubilityTemplate:Chembox BandGapTemplate:Chembox ThermalConductivityTemplate:Chembox RefractIndexTemplate:Chembox DipoleTemplate:Chembox StructureTemplate:Chembox ThermochemistryTemplate:Chembox MainHazardsTemplate:Chembox RPhrasesTemplate:Chembox NFPATemplate:Chembox FlashPtTemplate:Chembox NIOSH (set)Template:Chembox OtherAnionsTemplate:Chembox OtherCationsTemplate:Chembox Supplement| Template:Chembox header2 | Magnesium oxide | |
|---|---|
| Identifiers | |
| ChEMBL | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
PubChem CID
|
|
| Properties | |
| MgO | |
| Molar mass | 40.3044 g/mol |
| Hazards | |
| Related compounds | |
| Template:Chembox header2 | Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Mechanism of Action
Structure
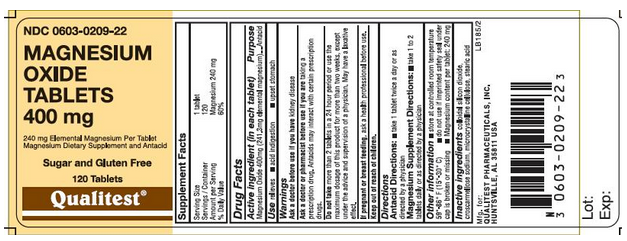
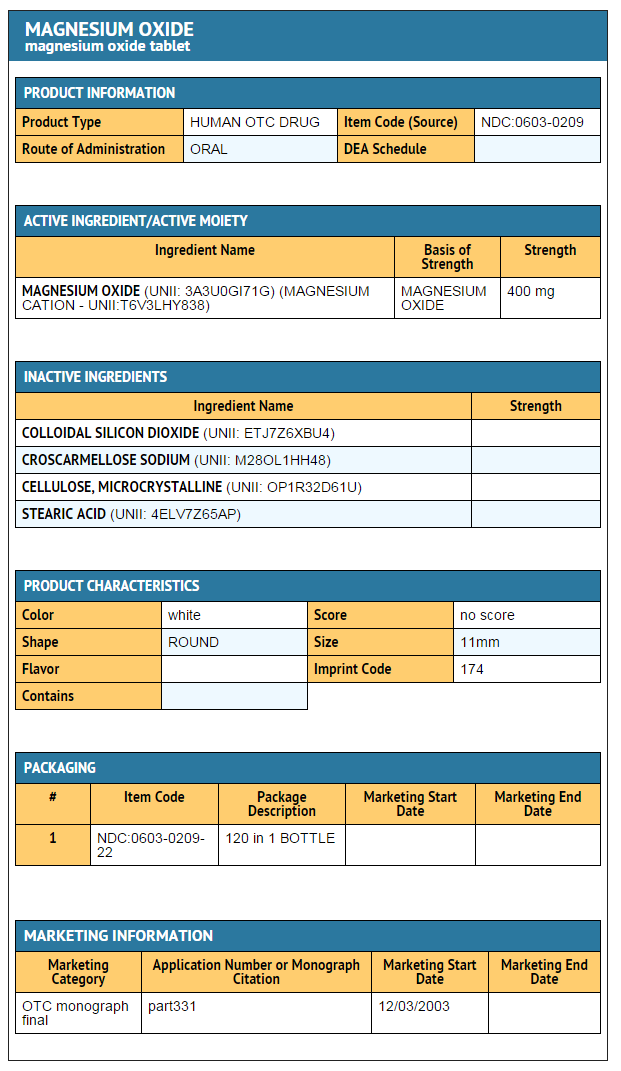
- ACTIVE INGREDIENTS
- Magnesium Oxide 400mg (241.3mg elemental magnesium)
- INACTIVE INGREDIENTS
- Colloidal silicon dioxide, croscarmellose sodium, microcrystalline cellulose, stearic acid
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Magnesium oxide in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Magnesium oxide in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Magnesium oxide in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Magnesium oxide in the drug label.
How Supplied
- do not use if imprinted safety seal under cap is broken or missing
- Magnesium content per tablet: 240 mg
Storage
- store at room temperature 59°-86° F (15°-30°C)
Images
Drug Images
{{#ask: Page Name::Magnesium oxide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL
Ingredients and Appearance
{{#ask: Label Page::Magnesium oxide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Magnesium oxide in the drug label.
Precautions with Alcohol
- Alcohol-Magnesium oxide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- MagGel®[4]
Look-Alike Drug Names
There is limited information regarding look alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Taurian, O.E.; Springborg, M.; Christensen, N.E. (1985). "Self-consistent electronic structures of MgO and SrO" (PDF). Solid State Communications. 55 (4): 351–5. Bibcode:1985SSCom..55..351T. doi:10.1016/0038-1098(85)90622-2.
- ↑ Application of magnesium compounds to insulating heat-conductive fillers. konoshima.co.jp
- ↑ 3.0 3.1 3.2 Template:PGCH
- ↑ "Magnesium oxide".