M2 protein
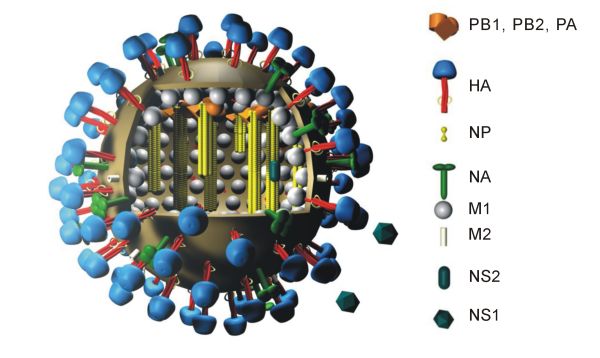
The M2 protein is a proton-selective ion channel protein, integral in the viral envelope of the influenza A virus. The channel itself is a homotetramer (consists of four identical M2 units), where the units are helixes stabilized by two disulfide bonds. It is activated by low pH.
Structure
The M2 protein unit consists of three protein domains: the 24 amino acids on the N-terminal end, exposed to the outside environment, the 19 hydrophobic aminoacids on the transmembrane region, and the 54 aminoacids on the C-terminal end, oriented towards the inside of the viral particle.
Function
The M2 protein has an important role in the life cycle of the influenza A virus. It is located in the viral envelope. It enables hydrogen ions to enter the viral particle (virion) from the endosome, thus lowering pH of the inside of the virus, which causes dissociation of the viral matrix protein M1 from the ribonucleoprotein RNP. This is a crucial step in uncoating of the virus and exposing its content to the cytoplasm of the host cell.
Inhibition and resistance
The function of the M2 channel can be inhibited by antiviral drugs amantadine and rimantadine, which then blocks the virus from taking over the host cell. The molecule of the drug binds to the transmembrane region, sterically blocking the channel. This stops the protons from entering the virion, which then does not disintegrate.
However, the M2 gene is susceptible to mutations. When one of five aminoacids in the transmembrane region gets suitably substituted, the virus gains resistance to the existing M2 inhibitors. As the mutations are relatively frequent, presence of the selection factors (eg. using amantadine for treatment of sick poultry) can lead to emergence of a resistant strain.
See also
Sources and notes
External links
- M2+protein,+Influenza+A+virus at the US National Library of Medicine Medical Subject Headings (MeSH)