Lopressor injection
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
For information about metoprolol, click here.
Disclaimer
WikiDoc Drug Project is a constellation of drug information for healthcare providers and patients vigorously vetted on the basis of FDA package insert, MedlinePlus, Practice Guidelines, Scientific Statements, and scholarly medical literature. The information provided is not a medical advice or treatment. WikiDoc does not promote any medication or off-label use of drugs. Please read our full disclaimer here.
Black Box Warning
FDA Package Insert for Lopressor injection contains no information regarding Black Box Warning.
Overview
Lopressor injection is a anti-anginal, antiarrhythmic, beta-adrenergic blocker drug that is FDA approved for the treatment of acute myocardial infarction (AMI). Common adverse reactions include bradyarrhythmia, constipation, depression, diarrhea, dizziness, dyspnea, fatigue, headache, heart block, heart failure, hypotension, indigestion, nausea, pruritus, rash, and wheezing.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acute Myocaridal Infarction
- Dosing Information
- Upon patient presentation: Lopressor injection 5 mg IV x 3 boluses at 2-min intervals
- 15 minutes after the full IV dose: Lopressor tablet 50 mg PO q6h for 2 days followed by Lopressor tablet 100 mg PO q12h (or Lopressor tablet 25—50 mg PO q6h for 2 days if intolerable to the full IV dose; discontinue Lopressor in patients with severe intolerance.)
Lopressor injection is indicated in the treatment of hemodynamically stable patients with definite or suspected acute myocardial infarction to reduce cardiovascular mortality when used in conjunction with oral Lopressor maintenance therapy. Treatment with Lopressor injection can be initiated as soon as the patient’s clinical condition allows.
Early Treatment: During the early phase of definite or suspected acute myocardial infarction, initiate treatment with Lopressor injection as soon as possible after the patient’s arrival in the hospital. Such treatment should be initiated in a coronary care or similar unit immediately after the patient’s hemodynamic condition has stabilized. Begin treatment in this early phase with the intravenous administration of three bolus injections of 5 mg of Lopressor injection each; give the injections at approximately 2-minute intervals. During the intravenous administration of Lopressor, monitor blood pressure, heart rate, and electrocardiogram.
In patients who tolerate the full intravenous dose (15 mg), initiate Lopressor tablets, 50 mg every 6 hours, 15 minutes after the last intravenous dose and continue for 48 hours. Thereafter, the maintenance dosage is 100 mg orally twice daily. Start patients who appear not to tolerate the full intravenous dose on Lopressor tablets either 25 mg or 50 mg every 6 hours (depending on the degree of intolerance) 15 minutes after the last intravenous dose or as soon as their clinical condition allows. In patients with severe intolerance, discontinue Lopressor (see Warnings).[1]
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Atrial Fibrillation, Rate Control
- Developed by: American College of Cardiology (ACC) and American Heart Association (AHA)
- Class of Recommendation: Class I
- Level of Evidence: Level B
- Dosing Information
- Acute Setting: Lopressor injection 2.5—5 mg IV bolus over 2 min; up to 3 doses[2]
In the absence of preexcitation, intravenous administration of beta blockers (esmolol, metoprolol, or propranolol) or nondihydropyridine calcium channel antagonists (verapamil, diltiazem) is recommended to slow the ventricular response to atrial fibrillation in the acute setting, exercising caution in patients with hypotension or heart failure.[3]
Non–Guideline-Supported Use
Atrial flutter
- Dosing Information
- Lopressor injection dose range: 2—15 mg[4]
Multifocal atrial tachycardia
- Dosing Information
- Lopressor injection dose range: 2—15 mg[4]
Supraventricular tachycardia
- Dosing Information
- Lopressor injection dose range: 2—15 mg[4]
Injection Site Pain Associated with Propofol Use
- Dosing Information
- Pretreatment with Lopressor injection 2 mg.[5]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
- Ventricular fibrillation
- Hypersensitivity to digoxin
Warnings
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Condition 1
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 1
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
(Description)
Labor and Delivery
(Description)
Nursing Mothers
(Description)
Pediatric Use
(Description)
Geriatric Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
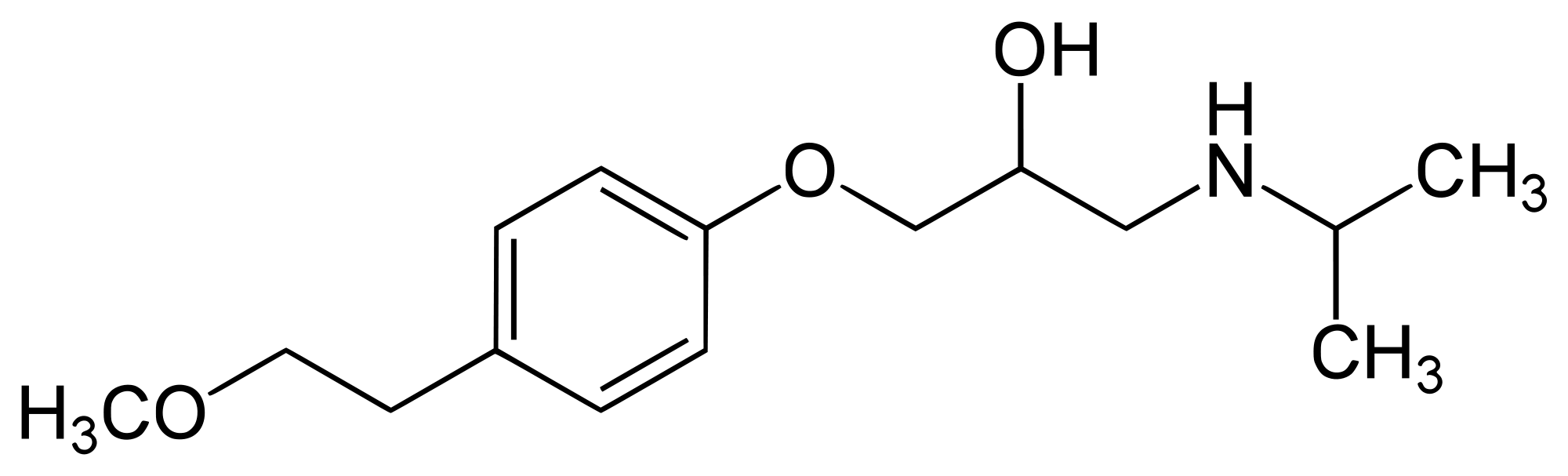
| |
Metoprolol
| |
| Systematic (IUPAC) name | |
| (RS)-1-(Isopropylamino)-3-[4-(2-methoxyethyl)phenoxy]propan-2-ol | |
| Identifiers | |
| CAS number | |
| ATC code | C07 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 267.364 g/mol |
| SMILES | & |
| Physical data | |
| Melt. point | 120 °C (248 °F) |
| Pharmacokinetic data | |
| Bioavailability | 12% |
| Metabolism | Hepatic via CYP2D6, CYP3A4 |
| Half life | 3-7 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. | |
| Legal status |
Template:Unicode Prescription only |
| Routes | Oral, IV |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
- National Drug Code (NDC):
- Storage:
- Manufactured by:
- Distributed by:
Images
Drug Images
(PillBox Images)
Package and Label Display Panel
(Package Images)
(Display Panel Images)
Patient Information
Patient Information from FDA
(Patient Counseling Information)
Patient Information from NLM
(Link to patient information page)
Precautions with Alcohol
Alcohol-Lopressor injection interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Price
References
- ↑ "LOPRESSOR (METOPROLOL TARTRATE) INJECTION, SOLUTION [NOVARTIS PHARMACEUTICALS CORPORATION]".
- ↑ Fuster, V.; Rydén, LE.; Cannom, DS.; Crijns, HJ.; Curtis, AB.; Ellenbogen, KA.; Halperin, JL.; Le Heuzey, JY.; Kay, GN. (2006). "ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation)". Eur Heart J. 27 (16): 1979–2030. doi:10.1093/eurheartj/ehl176. PMID 16885201. Unknown parameter
|month=ignored (help) - ↑ Wann, LS.; Curtis, AB.; Ellenbogen, KA.; Estes, NA.; Ezekowitz, MD.; Jackman, WM.; January, CT.; Lowe, JE.; Page, RL. (2013). "Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines". Circulation. 127 (18): 1916–26. doi:10.1161/CIR.0b013e318290826d. PMID 23545139. Unknown parameter
|month=ignored (help) - ↑ 4.0 4.1 4.2 Amsterdam, EA.; Kulcyski, J.; Ridgeway, MG. (1991). "Efficacy of cardioselective beta-adrenergic blockade with intravenously administered metoprolol in the treatment of supraventricular tachyarrhythmias". J Clin Pharmacol. 31 (8): 714–8. PMID 1880230. Unknown parameter
|month=ignored (help) - ↑ Aşik, I.; Yörükoğlu, D.; Gülay, I.; Tulunay, M. (2003). "Pain on injection of propofol: comparison of metoprolol with lidocaine". Eur J Anaesthesiol. 20 (6): 487–9. PMID 12803269. Unknown parameter
|month=ignored (help)