Hydroa vacciniforme
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Jesus Rosario Hernandez, M.D. [2]
Overview
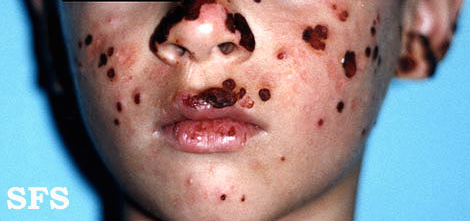
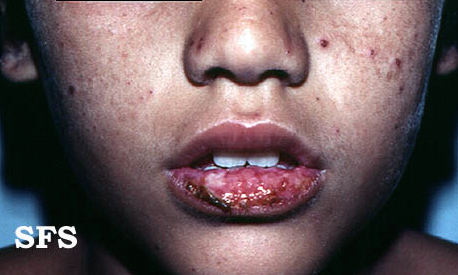
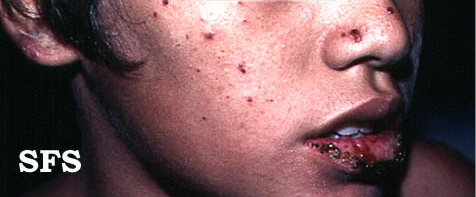
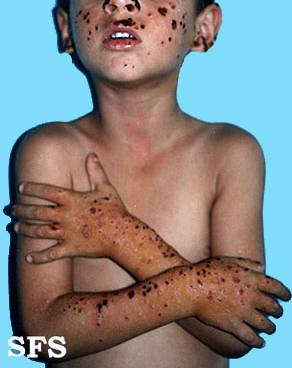
Hydroa vacciniforme' (HV) is a very rare, chronic photodermatitis-type skin condition with usual onset in childhood. It was first described in 1862 by Bazin.[1] It is sometimes called "Bazin's hydroa vacciniforme". A study published in Scotland in 2000 reviewed the cases of 17 patients and estimated a prevalence of 0.34 cases per 100,000 population. In this study they reported an average age of onset of 7.9 years. Frequently the rash first appeared in the spring or summer months and involved sun-exposed skin.[2] The rash starts as a vesicular eruption, later becoming umbilicated, and resulted in vacciniform scarring. It is most frequently found on the nose, cheeks, ears, dorsum of the hand, and arms (places that are most exposed to light).[3]
Natural History
In many children hydroa vacciniforme (HV) regresses spontaneously by early adulthood. In the 29 patients followed by Iwatuski et al, 11 of the 18 with definite or probable HV were available for follow-up and all were alive without progression of their symptoms. Some had recurrent eruptions of HV. In contrast out of 11 severe patients in this study, 6 had evidence of chronic EBV infection, 5 had hypersensitivity to mosquito bites, 4 had virus-associated hemophagocytic syndrome. 6 of the severe group had natural killer-cell lymphocytosis in the peripheral blood.
Physical examination
Gallery
Head
Trunk
Histology
Histology of the affected area commonly shows dense perivascular lymphocytic infiltration with reticulated degeneration of the epidermis. A study by Iwatsuki et al. detected Epstein-Barr virus (EBV) positive T-cells in the perivascular infiltration on biopsy in 28/29 patients tested. Antibody titers to EBV were measured in 14 of these patients and only five had abnormal antibody patterns consistent with chronic active EBV infection.[4]
Medical Therapy
Antiviral treatment has been tried with some success in a small number of patients.[5] In some cases, Heliocare Ultra-D capsules have proven to be a success in the treatment of the disease.
References
- ↑ Bazin, E (1862). "Lecons theoriques et cliniques sur les affectations generiques de la peau". Delabrage. 1:132.
- ↑ Gupta, G; Man I; Kemmett D. (2000). "Hydroa vacciniforme: A clinical and follow-up study of 17 cases". J Am Acad Dermatol. Feb;42(2 Pt 1): 208–13.
- ↑ James, William D.; Berger, Timothy G.; et al. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. ISBN 0-7216-2921-0.
- ↑ Iwatsuki, K; Satoh M; Yamamoto T; Oono T; Morizane S; Ohtsuka M; Xu ZG; Suzuki D; Tsuji K (2006). "Pathogenic link between hydroa vacciniforme and Epstein-Barr virus-associated hematologic disorders". Arch Dermatol. May;142(5): 587–95.
- ↑ Lysell, J (2009). "Antiviral therapy in children with hydroa vacciniforme". Acta Derm Venereol. 89 (4): 393–7. doi:10.2340/00015555-0670. Unknown parameter
|coauthors=ignored (help)