Herpes simplex virus
| Herpes simplex virus | ||||||||
|---|---|---|---|---|---|---|---|---|
 TEM micrograph of a herpes simplex virus.
| ||||||||
| Virus classification | ||||||||
| ||||||||
| Species | ||||||||
|
Herpes simplex virus 1 (HSV-1) |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]
Overview
Herpes simplex virus 1 and 2 (HSV-1 and HSV-2) are two strains of the herpes virus family, Herpesviridae, which cause extremely painful infections in humans.[1] They are also called Human Herpes Virus 1 and 2 (HHV-1 and HHV-2).
An infection is marked by watery blisters in the skin or mucous membranes (such as the mouth or lips) or on the genitals.[1] Lesions heal with a scab characteristic of herpetic disease. However, the infection is persistent and symptoms may recur periodically as outbreaks of sores near the site of original infection. After the initial, or primary, infection, HSV becomes latent present in the cell bodies of nerves in the area. When it becomes reactivated, it is produced in the nerve cell and transported to the skin via the nerve's axon.[2]
Herpes is contagious if the carrier is producing and shedding virus. This is especially likely during an outbreak but possible at other times. There is no cure yet, but there are treatments which reduce the likelihood of viral shedding. An HSV infection on the lips is commonly known as a "cold sore" or "fever blister". The blisters resemble those of chickenpox, an infection caused by another member of the alpha-Herpesviridae subfamily, Varicella Zoster Virus (VZV), also known as Human Herpes Virus 3 (HHV-3).
Transmission
HSV is transmitted by contact with lips or genitals when the sores are present, or possibly when sores are not visible (known as viral shedding). HSV can be present in semen, vaginal fluids, shedded skin in the pelvic region from eczema, and saliva. In addition, herpes may be transmitted during childbirth, which can be fatal to the infant. The immature immune system of the child is unable to defend against the virus and even if treated, infection can result in brain damage. Transmission occurs when passing through the birth canal. But, risk of infection is minimal if there are no symptoms or exposed blisters during delivery. The first outbreak after exposure to HSV is commonly more severe than future outbreaks, as the body has not had a chance to produce antibodies; this first outbreak carries a low (~1%) risk of developing aseptic meningitis.[1]
Microbiology
Viral structure
Animal herpes viruses all share some common properties. The structure of herpes viruses consists of a relatively large double-stranded, linear DNA genome encased within an icosahedral protein cage called the capsid, which is wrapped in a lipid bilayer called the envelope. The envelope is joined to the capsid by means of a tegument. This complete particle is known as the virion.[3] HSV-1 and HSV-2 each contain at least 74 genes (or open-reading frames, ORFs) within their genomes,[4] although speculation over gene crowding allows as many as 84 unique protein coding genes by 94 putative ORFs.[5] These genes encode a variety of proteins involved in forming the capsid, tegument and envelope of the virus, as well as controlling the replication and infectivity of the virus. These genes and their functions are summarized in the table below.
The genomes of HSV-1 and HSV-2 are complex, and contain two unique regions called the long unique region (UL) and the short unique region (US). Of the 74 known ORFs, UL contains 56 viral genes, whereas US contains only 12.[4] Transcription of HSV genes is catalyzed by RNA polymerase II of the infected host.[4] Immediate early genes, which encode proteins that regulate the expression of early and late viral genes, are the first to be expressed following infection. Early gene expression follows, to allow the synthesis of enzymes involved in DNA replication and the production of certain envelope glycoproteins. Expression of late genes occurs last; this group of genes predominantly encode proteins that form the virion particle.[4]
Five proteins from (UL) form the viral capsid; UL6, UL18, UL35, UL38 and the major capsid protein UL19.[3]
| The open reading frames (ORFs) of HSV-1[6][4] | |||||
| Gene | Protein | Function/description | Gene | Protein | Function/description |
| UL1 | Glycoprotein L [3] | Surface and membrane | UL38 | UL38; VP19C [4] | Capsid assembly and DNA maturation |
| UL2 | UL2 [5] | Uracil-DNA glycosylase | UL39 | UL39 [6] | Ribonucleotide reductase (Large subunit) |
| UL3 | UL3 [7] | unknown | UL40 | UL40 [8] | Ribonucleotide reductase (Small subunit) |
| UL4 | UL4 [9] | unknown | UL41 | UL41; VHS [10] | Tegument protein; Virion host shutoff[7] |
| UL5 | UL5 [11] | DNA replication | UL42 | UL42 [12] | DNA polymerase processivity factor |
| UL6 | UL6 [13] | Processing and packaging DNA | UL43 | UL43 [14] | Membrane protein |
| UL7 | UL7 [15] | Virion maturation | UL44 | Glycoprotein C [16] | Surface and membrane |
| UL8 | UL8 [17] | DNA helicase/primase complex-associated protein | UL45 | UL45 [18] | Membrane protein; C-type lectin[8] |
| UL9 | UL9 [19] | Replication origin-binding protein | UL46 | Alpha-TIF [20] | Tegument protein |
| UL10 | Glycoprotein M [21] | Surface and membrane | UL47 | UL47; VP13/14 [22] | Tegument protein |
| UL11 | UL11 [23] | virion exit and secondary envelopment | UL48 | ICP25; VP16 [24] | Virion maturation; activation of IEGs |
| UL12 | UL12 [25] | Alkaline exonuclease | UL49 | UL49A [26] | Envelope protein |
| UL13 | UL13 [27] | Serine-threonine protein kinase | UL50 | UL50 [28] | dUTP diphosphatase |
| UL14 | UL14 [29] | Tegument protein | UL51 | UL51 [30] | Tegument protein |
| UL15 | Terminase [31] | Processing and packaging of DNA | UL52 | UL52 [32] | DNA helicase/primase complex protein |
| UL16 | UL16 [33] | Tegument protein | UL53 | Glycoprotein K [34] | Surface and membrane |
| UL17 | UL17 [35] | Processing and packaging DNA | UL54 | IE63; ICP27 [36] | Transcriptional regulation |
| UL18 | VP23 [37] | Capsid protein | UL55 | UL55 [38] | Unknown |
| UL19 | VP5 [39] | Major capsid protein | UL56 | UL56 [40] | Unknown |
| UL20 | UL20 [41] | Membrane protein | US1 | ICP22; IE68 [42] | Viral replication |
| UL21 | UL21 [43] | Tegument protein[9] | US2 | US2 [44] | Unknown |
| UL22 | Glycoprotein H [45] | Surface and membrane | US3 | US3 [46] | Serine/threonine-protein kinase |
| UL23 | Thymidine kinase [47] | Peripheral to DNA replication | US4 | Glycoprotein G [48] | Surface and membrane |
| UL24 | UL24 [49] | unknown | US5 | Glycoprotein J [50] | Surface and membrane |
| UL25 | UL25 [51] | Processing and packaging DNA | US6 | Glycoprotein D [52] | Surface and membrane |
| UL26 | P40; VP24; VP22A [53] | Capsid protein | US7 | Glycoprotein I [54] | Surface and membrane |
| UL27 | Glycoprotein B [55] | Surface and membrane | US8 | Glycoprotein E [56] | Surface and membrane |
| UL28 | ICP18.5 [57] | Processing and packaging DNA | US9 | US9 [58] | Tegument protein |
| UL29 | UL29 [59] | Major DNA-binding protein | US10 | US10 [60] | Capsid/Tegument protein |
| UL30 | DNA polymerase [61] | DNA replication | US11 | US11; Vmw21 [62] | Binds DNA and RNA |
| UL31 | UL31 [63] | Nuclear matrix protein | US12 | ICP47; IE12 [64] | Inhibits MHC class I pathway |
| UL32 | UL32 [65] | Envelope glycoprotein | RS1 | ICP4; IE175 [66] | Activates gene transcription |
| UL33 | UL33 [67] | Processing and packaging DNA | ICP0 | ICP0; IE110; α0 [68] | Regulates gene transcription |
| UL34 | UL34 [69] | Inner nuclear membrane protein | LRP1 | LRP1 [70] | Latency-related protein |
| UL35 | VP26 [71] | Capsid protein | LRP2 | LRP2 [72] | Latency-related protein |
| UL36 | UL36 [73] | Large tegument protein | RL1 | RL1; ICP34.5 [74] | Neurovirulence factor |
| UL37 | UL37 [75] | Capsid assembly | LAT | none [76] | Latency-associated transcript |
Cellular entry
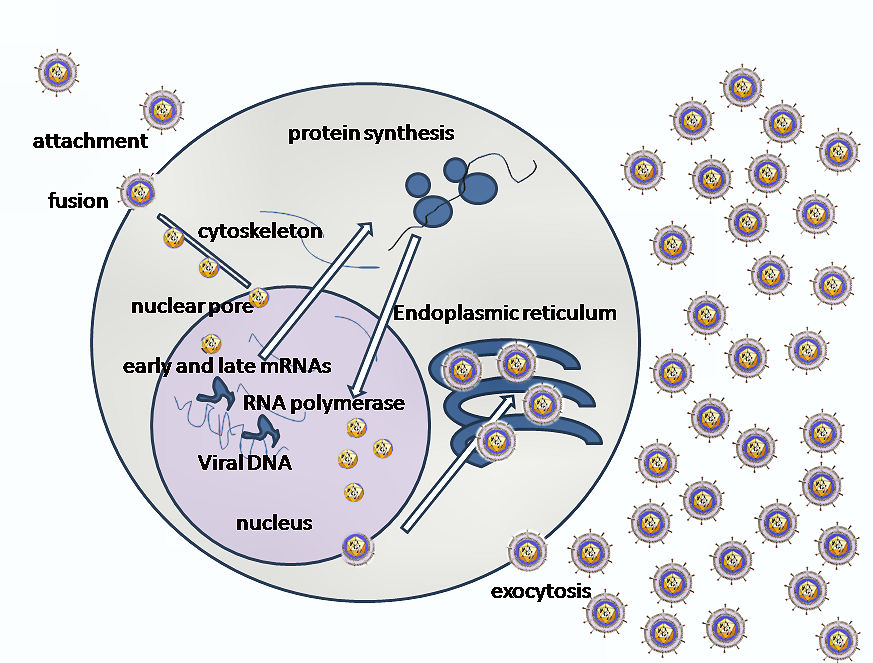
Entry of HSV into the host cell involves interactions of several glycoproteins on the surface of the enveloped virus, with receptors on the surface of the host cell. The envelope covering the virus particle, when bound to specific receptors on the cell surface, will fuse with the host cell membrane and create an opening, or pore, through which the virus enters the host cell.
The sequential stages of HSV entry are analogous to those of other viruses. At first, complementary receptors on the virus and the cell surface bring the viral and cell membranes into proximity. In an intermediate state, the two membranes begin to merge, forming a hemifusion state. Finally, a stable entry pore is formed through which the viral envelope contents are introduced to the host cell.[10] In the case of a herpes virus, initial interactions occur when a viral envelope glycoprotein called glycoprotein C (gC) binds to a cell surface particle called heparan sulfate. A second glycoprotein, glycoprotein D (gD), binds specifically to a receptor called the herpesvirus entry mediator receptor (HVEM) and provides a strong, fixed attachment to the host cell. These interactions bring the membrane surfaces into mutual proximity and allow for other glycoproteins embedded in the viral envelope to interact with other cell surface molecules.
Once bound to the HVEM, gD changes its conformation and interacts with viral glycoproteins H (gH) and L (gL), which form a complex. The interaction of these membrane proteins results in the hemifusion state. Afterward, gB interaction with the gH/gL complex creates an entry pore for the viral capsid.[10] Glycoprotein B interacts with glycosaminoglycans on the surface of the host cell.
Genetic inoculation
After the viral capsid enters the cellular cytoplasm, it is transported to the cell nucleus. Once attached to the nucleus at a nuclear entry pore, the capsid ejects its DNA contents via the capsid portal. The capsid portal is formed by twelve copies of portal protein arranged as a ring; the proteins contain a leucine zipper sequence of amino acids which allow them to adhere to each other.[11] Each icosahedral capsid contains a single portal, located in one vertex.[12][13] The DNA exits the capsid in a single linear segment.[14]
Replication
Consequent to a cell being infected, groups of Herpes virus proteins, termed immediate-early, early, and late proteins, are produced following specific time periods. Research using a new flow cytometry methodology in KSHV indicates the possibility of an additional lytic stage, delayed-late.[15] These stages of lytic infection, particularly late lytic, are distinct from the latency stage. For example, in the case of HSV-1, no protein products are detected during latency.
The early proteins transcribed are used in the regulation of genetic replication of the virus. On entering the cell, an α-TIF protein joins the viral particle and aids in immediate-early Transcription. The virion host shutoff protein (VHF-UL41) is very important to viral replication. This enzyme shuts off protein synthesis in the host, degrades host mRNA, helps in viral replication, and regulates gene expression of viral proteins. The viral genome immediately travels to the nucleus but the VHF protein remains in the cytoplasm.[16][17]
The late proteins transcribed are used in the capsid formation and forming the receptors on the surface of the virus. The packaging of the viral particles, which include the genome, core and the capsid, occur in the nucleus. In the nucleus, cleavage of genome concatemers occurs and these are placed into pre-formed capsids. HSV-1 undergoes a process of primary and secondary envelopment. It acquires a primary envelope by budding into the inner nuclear membrane. This then fuses with the outer nuclear membrane releasing a naked capsid into the cytoplasm. The virus acquires its final envelope by budding into cytoplasmic vesicles.[18]
Latent infection
HSV may persist in a quiescent but persistent form known as latent infection, notably in neural ganglia.[1] During latent infection of a cell, HSV express Latency Associated Transcript (LAT) RNA. LAT is known to regulate the host cell genome and interferes with natural cell death mechanisms. By maintaining the host cells, LAT expression preserves a reservoir for the virus, which allows later recurrences to produce further infections.
A protein found in neurons may bind to Herpes DNA and regulate latency. Recent studies have found that the Herpes DNA contains a sequence that is involved in silencing the expression of a gene associated with lytic infection, ICP4. The sequence contains elements which bind to human nerve cell protein factors: the human neuronal protein Neuronal Restrictive Silencing Factor (NRSF), and human Repressor Element Silencing Transcription Factor (REST). When the proteins are able to bind to the viral DNA elements, histone deacytalization occurs atop the ICP4 gene sequence.[19][20]
Reactivation
The virus can be reactivated due to the effects of other illnesses such as cold and influenza, excema, menstruation, emotional and physical stress, exposure to bright sunlight, gastric upset, fatigue or injury, consequently resulting in the appearance of surface sores.
Treatment
Antimicrobial Regimen
- Genital Herpes[21]
- 1.First Clinical Episode of Genital Herpes
- Preferred Regimen (1): Acyclovir 400 mg PO tid for 7–10 days
- Preferred Regimen (2): Acyclovir 200 mg PO five times a day for 7–10 days
- Preferred Regimen (3): Valacyclovir 1 g PO bid for 7–10 days
- Preferred Regimen (4): Famciclovir 250 mg PO tid for 7–10 days
- Note:Treatment can be extended if healing is incomplete after 10 days of therapy.
- 2.Established HSV-2 Infection
- 2.1 Suppressive Therapy for Recurrent Genital Herpes
- Preferred Regimen (1): Acyclovir 400 mg PO bid
- Preferred Regimen (2): Valacyclovir 500 mg PO qd
- Preferred Regimen (3): Valacyclovir 1 g PO qd
- Preferred Regimen (4): Famciclovir 250 mg PO bid
- Note(1):Daily therapy with Acyclovir for as long as 6 years and with Valacyclovir OR Famciclovir for 1 year
- Note(2):Valacyclovir 500 mg qd might be less effective than other Valacyclovir OR Acyclovir dosing regimens in persons who have very frequent recurrences (i.e., ≥10 episodes per year).
- 2.2 Episodic Therapy for Recurrent Genital Herpes
- Preferred Regimen (1): Acyclovir 400 mg PO tid for 5 days
- Preferred Regimen (2): Acyclovir 800 mg PO bid for 5 days
- Preferred Regimen (3): Acyclovir 800 mg PO tid for 2 days
- Preferred Regimen (4): Valacyclovir 500 mg PO bid for 3 days
- Preferred Regimen (5): Valacyclovir 1 g PO qd for 5 days
- Preferred Regimen (6): Famciclovir 125 mg PO bid for 5 days
- Preferred Regimen (7): Famciclovir 1 g PO bid for 1 day
- Preferred Regimen (8): Famciclovir 500 mg once, followed by 250 mg PO bid for 2 days
- 3. Severe Disease (disseminated infection, pneumonitis, or hepatitis) or CNS complications (e.g., meningoencephalitis).
- Preferred Regimen: Acyclovir 5–10 mg/kg IV q8h for 2–7 days or until clinical improvement is observed, followed by oral antiviral therapy to complete at least 10 days of total therapy. HSV encephalitis requires 21 days of intravenous therapy. Impaired renal function warrants an adjustment in acyclovir dosage.
- 4. Special Considerations
- 4.1 HIV Infection
- 4.1.1 Daily Suppressive Therapy in Persons with HIV
- Preferred Regimen (1): Acyclovir 400–800 mg PO bid /tid
- Preferred Regimen (2): Valacyclovir 500 mg PO bid
- Preferred Regimen (3): Famciclovir 500 mg PO bid
- 4.1.2 Episodic Infection in Persons with HIV
- Preferred Regimen (1): Acyclovir 400 mg PO tid for 5–10 days
- Preferred Regimen (2): Valacyclovir 1 g PO bid for 5–10 days
- Preferred Regimen (3): Famciclovir 500 mg PO bid for 5–10 days
- Note: For severe HSV disease, initiating therapy with Acyclovir 5–10 mg/kg IV q8 h might be necessary.
- 4.2 Genital Herpes in Pregnancy
- Suppressive therapy of pregnant women with recurrent genital herpes
- Preferred Regimen (1): Acyclovir 400–800 mg PO bid /tid
- Preferred Regimen (2): Valacyclovir 500 mg PO bid
- Note:Treatment recommended starting at 36 weeks of gestation.
- 4.3 Neonatal Herpes
- Known or suspected neonatal herpes
- Preferred Regimen: Acyclovir 20 mg/kg IV q 8 h
- Note (1): Treatment for 14 days if disease is limited to the skin and mucous membranes, or
- Note (2): Treatment for 21 days for disseminated disease and that involving the central nervous system.
- 4.4 Acyclovir-resistant genital herpes
- 4.5 Management of Sex Partners
- Preferred Regimen (1): Acyclovir 400 mg PO tid for 7–10 days
- Preferred Regimen (2): Acyclovir 200 mg PO five times a day for 7–10 days
- Preferred Regimen (3): Valacyclovir 1 g PO bid for 7–10 days
- Preferred Regimen (4): Famciclovir 250 mg PO tid for 7–10 days
- Note:The sex partners of persons who have genital herpes can benefit from evaluation and counseling. Symptomatic sex partners should be evaluated and treated
- 4.6 Allergy, Intolerance, and Adverse Reactions
- Allergic and other adverse reactions to oral Acyclovir, Valacyclovir, and Famciclovir are rare. Desensitization to acyclovir has been described.
Vaccine research
Herpevac, a vaccine for HSV-2 is currently (as of February 2007) undergoing clinical testing in women in the United States and Canada.[22][23] Previous studies have determined that this vaccine is approximately 70% effective in women, but does not prevent the disease in men.[24]
References
- ↑ 1.0 1.1 1.2 1.3 Ryan KJ, Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed. ed.). McGraw Hill. pp. 555&ndash, 62. ISBN 0838585299.
- ↑ "Herpes simplex". DermNet NZ — New Zealand Dermatological Society. 2006-09-16. Retrieved 2006-10-15.
- ↑ 3.0 3.1 Mettenleiter TC, Klupp BG, Granzow H (2006). "Herpesvirus assembly: a tale of two membranes". Curr. Opin. Microbiol. 9 (4): 423–9. doi:10.1016/j.mib.2006.06.013. PMID 16814597.
- ↑ 4.0 4.1 4.2 4.3 4.4 McGeoch DJ, Rixon FJ, Davison AJ (2006). "Topics in herpesvirus genomics and evolution". Virus Res. 117 (1): 90–104. doi:10.1016/j.virusres.2006.01.002. PMID 16490275.
- ↑ Rajcáni J, Andrea V, Ingeborg R (2004). "Peculiarities of herpes simplex virus (HSV) transcription: an overview". Virus Genes. 28 (3): 293–310. PMID 15266111.
- ↑ Search in UniProt Knowledgebase (Swiss-Prot and TrEMBL) for: HHV1
- ↑ Matis J, Kúdelová M (2001). "Early shutoff of host protein synthesis in cells infected with herpes simplex viruses". Acta Virol. 45 (5–6): 269–77. PMID 12083325.
- ↑ Wyrwicz LS, Ginalski K, Rychlewski L (2007). "HSV-1 UL45 encodes a carbohydrate binding C-type lectin protein". Cell Cycle. 7 (2). PMID 18256535.
- ↑ Vittone V, Diefenbach E, Triffett D, Douglas MW, Cunningham AL, Diefenbach RJ (2005). "Determination of interactions between tegument proteins of herpes simplex virus type 1". J. Virol. 79 (15): 9566–71. doi:10.1128/JVI.79.15.9566-9571.2005. PMID 16014918.
- ↑ 10.0 10.1 Subramanian RP, Geraghty RJ (2007). "Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B". Proc. Natl. Acad. Sci. U.S.A. 104 (8): 2903–8. doi:10.1073/pnas.0608374104. PMID 17299053.
- ↑
Cardone G, Winkler DC, Trus BL, Cheng N, Heuser JE, Newcomb WW, Brown JC, Steven AC (2007 May 10). "Visualization of the herpes simplex virus portal in situ by cryo-electron tomography". Virology. 361 (2): 426-34. PMID 17188319. Check date values in:
|date=(help) - ↑ Trus BL, Cheng N, Newcomb WW, Homa FL, Brown JC, Steven AC (2004 Nov). "Structure and polymorphism of the UL6 portal protein of herpes simplex virus type 1". Journal of Virology. 78 (22): 12668-71. PMID 15507654. Check date values in:
|date=(help)(Article: [1]) - ↑
Nellissery JK, Szczepaniak R, Lamberti C, Weller SK (2007 Jun 20). "A putative leucine zipper within the HSV-1 UL6 protein is required for portal ring formation". Journal Virology. PMID 17581990. Check date values in:
|date=(help) - ↑ Newcomb WW, Booy FP, Brown JC (2007). "Uncoating the herpes simplex virus genome". J. Mol. Biol. 370 (4): 633–42. doi:10.1016/j.jmb.2007.05.023. PMID 17540405.
- ↑ Adang LA, Parsons CH, Kedes DH (2006). "Asynchronous progression through the lytic cascade and variations in intracellular viral loads revealed by high-throughput single-cell analysis of Kaposi's sarcoma-associated herpesvirus infection". J. Virol. 80 (20): 10073–82. doi:10.1128/JVI.01156-06. PMID 17005685.
- ↑ Taddeo B, Roizman B (2006). "The virion host shutoff protein (UL41) of herpes simplex virus 1 is an endoribonuclease with a substrate specificity similar to that of RNase A". J. Virol. 80 (18): 9341–5. doi:10.1128/JVI.01008-06. PMID 16940547.
- ↑ Skepper JN, Whiteley A, Browne H, Minson A(2001)."Herpes Simplex Virus Nucleocapsids Mature to Progeny Virions by an Envelopment-Deenvelopment-Reenvelopment Pathway".J.Virol.75(12):5697-5702
- ↑ Granzow H, Klupp BG, Fuchs W, Veits J, Osterrieder N, Mettenleiter TC (2001)."Egress of Alphaherpesviruses:Comparative Ultrastructural Study". J. Virol.75(8):3675-3684
- ↑ Pinnoji RC, Bedadala GR, George B, Holland TC, Hill JM, Hsia SC (2007). "Repressor element-1 silencing transcription factor/neuronal restrictive silencer factor (REST/NRSF) can regulate HSV-1 immediate-early transcription via histone modification". Virol. J. 4: 56. doi:10.1186/1743-422X-4-56. PMID 17555596.
- ↑ Bedadala GR, Pinnoji RC, Hsia SC (2007). "Early growth response gene 1 (Egr-1) regulates HSV-1 ICP4 and ICP22 gene expression". Cell Res. 17 (6): 546–55. doi:10.1038/cr.2007.44. PMID 17502875.
- ↑ Workowski, Kimberly A.; Bolan, Gail A. (2015-06-05). "Sexually transmitted diseases treatment guidelines, 2015". MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 64 (RR-03): 1–137. ISSN 1545-8601. PMID 26042815.
- ↑ Baker T (2006-06-13). "First herpes vaccine under study". Press release. Medical College of Georgia. Retrieved 2007-06-17.
- ↑ "Herpevac Trial for Women". NIH. Retrieved 2007-07-09.
- ↑ "Major Herpes Vaccine Trial Launched in Women". 2002-11-20. Retrieved 2007-07-09.
de:Herpes-simplex-Virus dv:ޖެނިޓަލް ހާރޕީޒް hr:Herpes simpleks id:Virus herpes simpleks it:Herpes simplex nl:Herpes simplexvirus no:Herpes simplex simple:Herpes simplex sl:Virus herpesa simpleksa fi:Herpes simplex sv:Herpes simplex ta:பாலுறுப்பு ஹேர்பீஸ் th:เริม