Foscarnet sodium
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
* RENAL IMPAIRMENT IS THE MAJOR TOXICITY OF FOSCAVIR. FREQUENT MONITORING OF SERUM CREATININE, WITH DOSE ADJUSTMENT FOR CHANGES IN RENAL FUNCTION, AND ADEQUATE HYDRATION WITH ADMINISTRATION OF FOSCAVIR IS IMPERATIVE.
|
Overview
Foscarnet sodium is an antiviral medication that is FDA approved for the treatment of CMV retinitis, mucocutaneous acyclovir resistant HSV infections. There is a Black Box Warning for this drug as shown here. Common adverse reactions include fever, nausea, diarrhea, anemia, abdominal pain,hypokalemia, coughing, dyspnea,confusion, anxiety.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
CMV Retinitis
- Foscarnet sodium is indicated for the treatment of CMV retinitis in patients with acquired immunodeficiency syndrome (AIDS). Combination therapy with FOSCARNET SODIUM and ganciclovir is indicated for patients who have relapsed after monotherapy with either drug. SAFETY AND EFFICACY OF FOSCARNET SODIUM HAVE NOT BEEN ESTABLISHED FOR TREATMENT OF OTHER CMV INFECTIONS (e.g., PNEUMONITIS, GASTROENTERITIS); CONGENITAL OR NEONATAL CMV DISEASE; OR NONIMMUNOCOMPROMISED INDIVIDUALS.
Mucocutaneous Acyclovir Resistant HSV Infections
- Foscarnet sodium is indicated for the treatment of acyclovir-resistant mucocutaneous HSV infections in immunocompromised patients. SAFETY AND EFFICACY OF FOSCAVIR HAVE NOT BEEN ESTABLISHED FOR TREATMENT OF OTHER HSV INFECTIONS (e.g., RETINITIS, ENCEPHALITIS); CONGENITAL OR NEONATAL HSV DISEASE; OR HSV IN NONIMMUNOCOMPROMISED INDIVIDUALS.
Dosage
THE RECOMMENDED DOSAGE, FREQUENCY, OR INFUSION RATES SHOULD NOT BE EXCEEDED. ALL DOSES MUST BE INDIVIDUALIZED FOR PATIENTS' RENAL FUNCTION.
Induction Treatment
- The recommended initial dose of foscarnet sodium for patients with normal renal function is:
- For CMV retinitis patients, either 90 mg/kg (1-1/2 to 2 hour infusion) every twelve hours or 60 mg/kg (minimum one hour infusion) every eight hours over 2-3 weeks depending on clinical response.
- For acyclovir-resistant HSV patients, 40 mg/kg (minimum one hour infusion) either every 8 or 12 hours for 2-3 weeks or until healed.
- An infusion pump must be used to control the rate of infusion. Adequate hydration is recommended to establish a diuresis (see HYDRATION FOR RECOMMENDATION), both prior to and during treatment to minimize renal toxicity, provided there are no clinical contraindications.
Maintenance Treatment
- Following induction treatment the recommended maintenance dose of foscarnet sodium for CMV retinitis is 90 mg/kg/day to 120 mg/kg/day (individualized for renal function) given as an intravenous infusion over 2 hours. Because the superiority of the 120 mg/kg/day has not been established in controlled trials, and given the likely relationship of higher plasma foscarnet levels to toxicity, it is recommended that most patients be started on maintenance treatment with a dose of 90 mg/kg/day. Escalation to 120 mg/kg/day may be considered should early re induction be required because of retinitis progression. Some patients who show excellent tolerance to foscarnet sodium may benefit from initiation of maintenance treatment at 120 mg/kg/day earlier in their treatment.
- An infusion pump must be used to control the rate of infusion with all doses. Again, hydration to establish diuresis both prior to and during treatment is recommended to minimize renal toxicity, provided there are no clinical contraindications.
- Patients who experience progression of retinitis while receiving foscarnet sodium maintenance therapy may be retreated with the induction and maintenance regimens given above or with a combination of foscarnet sodium and ganciclovir. Because of physical incompatibility, foscarnet sodium and ganciclovir must not be mixed.
Use in Patients with Abnormal Renal Function
- foscarnet sodium should be used with caution in patients with abnormal renal function because reduced plasma clearance of foscarnet will result in elevated plasma levels. In addition, foscarnet sodium has the potential to further impair renal function. Safety and efficacy data for patients with baseline serum creatinine levels greater than 2.8 mg/dL or measured 24-hour creatinine clearances < 50 mL/min are limited.
- Renal function must be monitored carefully at baseline and during induction and maintenance therapy with appropriate dose adjustments for foscarnet sodium as outlined below .During foscarnet sodium therapy if creatinine clearance falls below the limits of the dosing nomograms (0.4 mL/min/kg), foscarnet sodium should be discontinued, the patient hydrated, and monitored daily until resolution of renal impairment is ensured.
- Foscarnet sodium is not recommended in patients undergoing hemodialysis because dosage guidelines have not been established.
Dose Adjustment
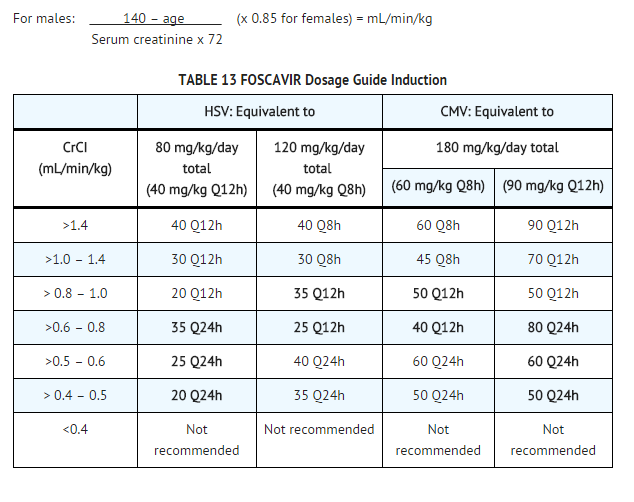
- Foscarnet sodium dosing must be individualized according to the patient's renal function status. Refer to TABLE 13 below for recommended doses and adjust the dose as indicated. Even patients with serum creatinine in the normal range may require dose adjustment; therefore, the dose should be calculated at baseline and frequently thereafter.
- To use this dosing guide, actual 24-hour creatinine clearance (mL/min) must be divided by body weight (kg), or the estimated creatinine clearance in mL/min/kg can be calculated from serum creatinine (mg/dL) using the following formula (modified Cockcroft and Gault equation):

Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Foscarnet sodium in adult patients.
Non–Guideline-Supported Use
Indication
- Cytomegaloviral pneumonia
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Foscarnet sodium in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Foscarnet sodium in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Foscarnet sodium in pediatric patients.
Contraindications
- FOSCAVIR is contraindicated in patients with clinically significant hypersensitivity to foscarnet sodium.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
* RENAL IMPAIRMENT IS THE MAJOR TOXICITY OF FOSCAVIR. FREQUENT MONITORING OF SERUM CREATININE, WITH DOSE ADJUSTMENT FOR CHANGES IN RENAL FUNCTION, AND ADEQUATE HYDRATION WITH ADMINISTRATION OF FOSCAVIR IS IMPERATIVE.
|
Renal Impairment
- THE MAJOR TOXICITY OF FOSCARNET SODIUM IS RENAL IMPAIRMENT. Renal impairment is most likely to become clinically evident during the second week of induction therapy, but may occur at any time during foscarnet sodium treatment. Renal function should be monitored carefully during both induction and maintenance therapy. Elevations in serum creatinine are usually, but not always, reversible following discontinuation or dose adjustment of FOSCAVIR. Safety and efficacy data for patients with baseline serum creatinine levels greater than 2.8 mg/dL or measured 24-hour creatinine clearances <50 mL/min are limited.
- SINCE FOSCARNET SODIUM HAS THE POTENTIAL TO CAUSE RENAL IMPAIRMENT, DOSE ADJUSTMENT BASED ON SERUM CREATININE IS NECESSARY. Hydration may reduce the risk of nephrotoxicity. It is recommended that 750–1000 mL of normal saline or 5% dextrose solution should be given prior to the first infusion of foscarnet sodium to establish diuresis. With subsequent infusions, 750–1000 mL of hydration fluid should be given with 90-120 mg/kg of foscarnet sodium, and 500 mL with 40–60 mg/kg of foscarnet sodium. Hydration fluid may need to be decreased if clinically warranted.
- After the first dose, the hydration fluid should be administered concurrently with each infusion of foscarnet sodium.
Mineral and Electrolyte Abnormalities
- Foscarnet sodium has been associated with changes in serum electrolytes including hypocalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia, and hypokalemia. Foscarnet sodium may also be associated with a dose-related decrease in ionized serum calcium which may not be reflected in total serum calcium. This effect is likely to be related to chelation of divalent metal ions such as calcium by foscarnet. Patients should be advised to report symptoms of low ionized calcium such as perioral tingling, numbness in the extremities and paresthesias. Particular caution and careful management of serum electrolytes is advised in patients with altered calcium or other electrolyte levels before treatment and especially in those with neurologic or cardiac abnormalities and those receiving other drugs known to influence minerals and electrolytes . Physicians should be prepared to treat these abnormalities and their sequelae such as tetany, seizures or cardiac disturbances. The rate of foscarnet sodium infusion may also affect the decrease in ionized calcium. Therefore, an infusion pump must be used for administration to prevent rapid intravenous infusion. Slowing the infusion rate may decrease or prevent symptoms.
Seizures
- Seizures related to mineral and electrolyte abnormalities have been associated with foscarnet sodium treatment. Several cases of seizures were associated with death. Risk factors associated with seizures included impaired baseline renal function, low total serum calcium, and underlying CNS conditions.
Adverse Reactions
Clinical Trials Experience
- THE MAJOR TOXICITY OF FOSCAVIR IS RENAL IMPAIRMENT. Approximately 33% of 189 patients with AIDS and CMV retinitis who received foscarnet sodium (60 mg/kg TID), without adequate hydration, developed significant impairment of renal function (serum creatinine ≥ 2.0 mg/dL). The incidence of renal impairment in subsequent clinical trials in which 1000 mL of normal saline or 5% dextrose solution was given with each infusion of foscarnet sodium was 12% (34/280).
- Foscarnet sodium has been associated with changes in serum electrolytes including hypocalcemia (15-30%), hypophosphatemia (8–26%) and hyperphosphatemia (6%), hypomagnesemia (15–30%), and hypokalemia (16–48%). The higher percentages were derived from those patients receiving hydration.
- Foscarnet sodium treatment was associated with seizures in 18/189 (10%) AIDS patients in the initial five controlled studies. Risk factors associated with seizures included impaired baseline renal function, low total serum calcium, and underlying CNS conditions predisposing the patient to seizures. The rate of seizures did not increase with duration of treatment. Three cases were associated with overdoses of foscarnet sodium.
- In five controlled U.S. clinical trials the most frequently reported adverse events in patients with AIDS and CMV retinitis are shown in TABLE 9. These figures were calculated without reference to drug relationship or severity.

- From the same controlled studies, adverse events categorized by investigator as “severe” are shown in TABLE 10. Although death was specifically attributed to foscarnet sodium in only one case, other complications of foscarnet sodium (i.e., renal impairment, electrolyte abnormalities, and seizures) may have contributed to patient deaths.

- From the five initial U.S. controlled trials of foscarnet sodium, the following list of adverse events has been compiled regardless of causal relationship to foscarnet sodium. Evaluation of these reports was difficult because of the diverse manifestations of the underlying disease and because most patients received numerous concomitant medications.
Incidence of 5% or Greater
- Central and Peripheral Nervous System: headache, paresthesia, dizziness, involuntary muscle contractions, hypoesthesia, neuropathy, seizures including grand mal seizures
- Gastrointestinal System: anorexia, nausea, diarrhea, vomiting, abdominal pain
- Hematologic: anemia, granulocytopenia, leukopenia, neutropenia
- Metabolic and Nutritional: mineral and electrolyte imbalances including hypokalemia, hypocalcemia, hypomagnesemia, hypophosphatemia, hyperphosphatemia
- Psychiatric: depression, confusion, anxiety
- Respiratory System: coughing, dyspnea
- Skin and Appendages: rash, increased sweating
- Urinary: alterations in renal function including increased serum creatinine, decreased creatinine clearance, and abnormal renal function
- Special Senses: vision abnormalities
- Urinary System: albuminuria, dysuria, polyuria, urethral disorder, urinary retention, urinary tract infections, acute renal failure, nocturia, facial edema
- Selected adverse events occurring at a rate of less than 1% in the five initial U.S. controlled clinical trials of foscarnet sodium include: syndrome of inappropriate antidiuretic hormone secretion, pancytopenia, hematuria, dehydration, hypoproteinemia, increases in amylase and creatinine phosphokinase, cardiac arrest, coma, and other cardiovascular and neurologic complications.
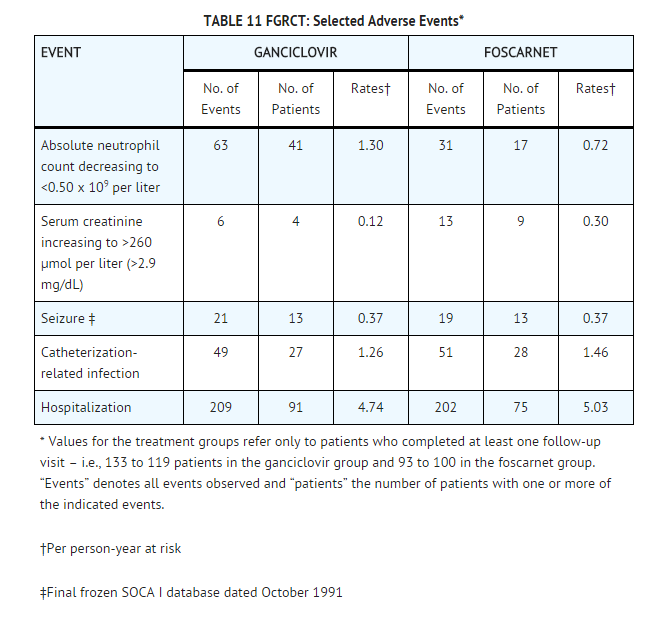
- Selected adverse event data from the Foscarnet vs. Ganciclovir CMV Retinitis Trial (FGCRT), performed by the Studies of the Ocular Complications of AIDS (SOCA) Research Group, are shown in TABLE 11.
- Selected adverse events from ACTG Study 228 (CRRT) comparing combination therapy with foscarnet sodium or ganciclovir monotherapy are shown in TABLE 12. The most common reason for a treatment change in patients assigned to either foscarnet sodium or ganciclovir was retinitis progression. The most frequent reason for a treatment change in the combination treatment group was toxicity.

Postmarketing Experience
- Adverse events that have been reported in post-marketing surveillance include: ventricular arrhythmia, prolongation of QT interval, gamma GT increased, diabetes insipidus (usually nephrogenic), renal calculus, esophageal ulceration, renal tubular acidosis, renal tubular necrosis, crystal-induced nephropathy and muscle disorders including myopathy, myositis, muscle weakness and rare cases of rhabdomyolysis. Cases of vesiculobullous eruptions including erythema multiforme, toxic epidermal necrolysis, and Stevens-Johnson Syndrome have been reported. In most cases, patients were taking other medications that have been associated with toxic epidermal necrolysis or Stevens-Johnson Syndrome.
Drug Interactions
- A possible drug interaction of foscarnet sodium and intravenous pentamidine has been described. Concomitant treatment of four patients in the United Kingdom with foscarnet sodium and intravenous pentamidine may have caused hypocalcemia; one patient died with severe hypocalcemia. Toxicity associated with concomitant use of aerosolized pentamidine has not been reported. Because foscarnet sodium can reduce serum levels of ionized calcium, extreme caution is advised when used concurrently with other drugs known to influence serum calcium levels (e.g., intravenous pentamidine). Renal impairment and symptomatic hypocalcemia have been observed during concurrent treatment with foscarnet sodium and intravenous pentamidine.
- Because of foscarnet's tendency to cause renal impairment, the use of FOSCAVIR should be avoided in combination with potentially nephrotoxic drugs such as aminoglycosides, amphotericin B, cyclosporine, acyclovir, methotrexate, tacrolimus and intravenous pentamidine unless the potential benefits outweigh the risks to the patient.
- When diuretics are indicated, thiazides are recommended over loop diuretics because the latter inhibit renal tubular secretion, and may impair elimination of foscarnet sodium, potentially leading to toxicity.
- Abnormal renal function has been observed in clinical practice during the use of foscarnet sodium and ritonavir, or foscarnet sodium, ritonavir, and saquinavir.
Use in Specific Populations
Pregnancy
- Pregnancy, Category C: There are no adequate and well-controlled studies of foscarnet sodium in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Animal Data: foscarnet sodium did not adversely affect fertility and general reproductive performance in rats. The results of peri- and post-natal studies in rats were also negative. However, these studies used exposures that are inadequate to define the potential for impairment of fertility at human drug exposure levels.
- Daily subcutaneous doses up to 75 mg/kg administered to female rats prior to and during mating, during gestation, and 21 days post-partum caused a slight increase (< 5%) in the number of skeletal anomalies compared with the control group. Daily subcutaneous doses up to 75 mg/kg administered to rabbits and 150 mg/kg administered to rats during gestation caused an increase in the frequency of skeletal anomalies/variations. On the basis of estimated drug exposure (as measured by AUC), the 150 mg/kg dose in rats and 75 mg/kg dose in rabbits were approximately one-eighth (rat) and one-third (rabbit) the estimated maximal daily human exposure. These studies are inadequate to define the potential teratogenicity at levels to which women will be exposed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Foscarnet sodium in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Foscarnet sodium during labor and delivery.
Nursing Mothers
- It is not known whether foscarnet sodium is excreted in human milk; however, in lactating rats administered 75 mg/kg, foscarnet sodium was excreted in maternal milk at concentrations three times higher than peak maternal blood concentrations. Because of the potential for serious adverse events in nursing infants, a decision should be made whether to discontinue nursing or discontinue drug, taking into consideration the importance of the drug to the mother. The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breast-feed their infants to avoid risking postnatal transmission of HIV.
Pediatric Use
- The safety and effectiveness of foscarnet sodium in pediatric patients have not been established. foscarnet sodium is deposited in teeth and bone and deposition is greater in young and growing animals. foscarnet sodium has been demonstrated to adversely affect development of tooth enamel in mice and rats. The effects of this deposition on skeletal development have not been studied. Since deposition in human bone has also been shown to occur, it is likely that it does so to a greater degree in developing bone in pediatric patients. Administration to pediatric patients should be undertaken only after careful evaluation and only if the potential benefits for treatment outweigh the risks.
Geriatic Use
- No studies of the efficacy or safety of foscarnet sodium in persons 65 years of age or older have been conducted. However, foscarnet sodium has been used in patients age 65 years of age and older. The pattern of adverse events seen in these patients is consistent across all age groups. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and renal function should be monitored.
Gender
There is no FDA guidance on the use of Foscarnet sodium with respect to specific gender populations.
Race
There is no FDA guidance on the use of Foscarnet sodium with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Foscarnet sodium in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Foscarnet sodium in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Foscarnet sodium in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Foscarnet sodium in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
'CAUTION—DO NOT ADMINISTER FOSCARNET SODIUM BY RAPID OR BOLUS INTRAVENOUS INJECTION. THE TOXICITY OF FOSCARNET SODIUM MAY BE INCREASED AS A RESULT OF EXCESSIVE PLASMA LEVELS. CARE SHOULD BE TAKEN TO AVOID UNINTENTIONAL OVERDOSE BY CAREFULLY CONTROLLING THE RATE OF INFUSION. THEREFORE, AN INFUSION PUMP MUST BE USED. IN SPITE OF THE USE OF AN INFUSION PUMP, OVERDOSES HAVE OCCURRED.
Monitoring
- The majority of patients will experience some decrease in renal function due to foscarnet sodium administration. Therefore it is recommended that creatinine clearance, either measured or estimated using the modified Cockcroft and Gault equation based on serum creatinine, be determined at baseline, 2–3 times per week during induction therapy and once weekly during maintenance therapy, with foscarnet sodium dose adjusted accordingly. More frequent monitoring may be required for some patients. It is also recommended that a 24-hour creatinine clearance be determined at baseline and periodically thereafter to ensure correct dosing (assuming verification of an adequate collection using creatinine index). Foscarnet sodium should be discontinued if creatinine clearance drops below 0.4 mL/min/kg.
- Due to foscarnet sodium propensity to chelate divalent metal ions and alter levels of serum electrolytes, patients must be monitored closely for such changes. It is recommended that a schedule similar to that recommended for serum creatinine (see above) be used to monitor serum calcium, magnesium, potassium and phosphorus. Particular caution is advised in patients with decreased total serum calcium or other electrolyte levels before treatment, as well as in patients with neurologic or cardiac abnormalities, and in patients receiving other drugs known to influence serum calcium levels. Any clinically significant metabolic changes should be corrected. Also, patients who experience mild (e.g., perioral numbness or paresthesias) or severe (e.g., seizures) symptoms of electrolyte abnormalities should have serum electrolyte and mineral levels assessed as close in time to the event as possible.
- Careful monitoring and appropriate management of electrolytes, calcium, magnesium and creatinine are of particular importance in patients with conditions that may predispose them to seizures.
IV Compatibility
There is limited information regarding IV Compatibility of Foscarnet sodium in the drug label.
Overdosage
- In controlled clinical trials performed in the United States, overdosage with foscarnet sodium was reported in 10 out of 189 patients. All 10 patients experienced adverse events and all except one made a complete recovery. One patient died after receiving a total daily dose of 12.5 g for three days instead of the intended 10.9 g. The patient suffered a grand mal seizure and became comatose. Three days later the patient expired with the cause of death listed as respiratory/cardiac arrest. The other nine patients received doses ranging from 1.14 times to 8 times their recommended doses with an average of 4 times their recommended doses. Overall, three patients had seizures, three patients had renal function impairment, four patients had paresthesias either in limbs or periorally, and five patients had documented electrolyte disturbances primarily involving calcium and phosphate.
- Overdose (up to 20 times the recommended dose) has been reported in post-marketing use of foscarnet sodium. Some of these post-marketing reports were relative overdoses in that the dose of foscarnet sodium had not been adjusted in patients with a reduced renal function. The pattern of adverse events associated with a foscarnet sodium overdose is consistent with the known adverse event profile of the drug.
- There is no specific antidote for foscarnet sodium overdose. Hemodialysis and hydration may be of benefit in reducing drug plasma levels in patients who receive an overdosage of FOSCAVIR, but the effectiveness of these interventions has not been evaluated. The patient should be observed for signs and symptoms of renal impairment and electrolyte imbalance. Medical treatment should be instituted if clinically warranted.
Pharmacology

Mechanism of Action
There is limited information regarding Foscarnet sodium Mechanism of Action in the drug label.
Structure
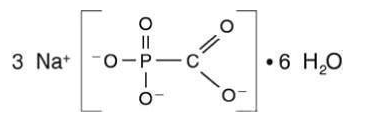
- FOSCAVIR is the brand name for foscarnet sodium. The chemical name of foscarnet sodium is phosphonoformic acid, trisodium salt. Foscarnet sodium is a white, crystalline powder containing 6 equivalents of water of hydration with an empirical formula of Na3CO5P•6 H20 and a molecular weight of 300.1. The structural formula is:
- FOSCAVIR has the potential to chelate divalent metal ions, such as calcium and magnesium, to form stable coordination compounds. FOSCAVIR INJECTION is a sterile, isotonic aqueous solution for intravenous administration only. The solution is clear and colorless. Each milliliter of FOSCAVIR contains 24 mg of foscarnet sodium hexahydrate in Water for Injection, USP. Hydrochloric acid may have been added to adjust the pH of the solution to 7.4. FOSCAVIR INJECTION contains no preservatives.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Foscarnet sodium in the drug label.
Pharmacokinetics
- The pharmacokinetics of foscarnet has been determined after administration as an intermittent intravenous infusion during induction therapy in AIDS patients with CMV retinitis. Observed plasma foscarnet concentrations in four studies (FOS-01, ACTG-015, FP48PK, FP49PK) are summarized in TABLE 7:

Distribution
- In vitro studies have shown that 14 – 17% of foscarnet is protein bound at plasma drug concentrations of 1 – 1000 μM.
- The foscarnet terminal half-life determined by urinary excretion was 87.5 ± 41.8 hours, possibly due to release of foscarnet from bone. Postmortem data on several patients in European clinical trials provide evidence that foscarnet does accumulate in bone in humans; however, the extent to which this occurs has not been determined.
Special Populations
- Adults with Impaired Renal Function: The pharmacokinetic properties of foscarnet have been determined in a small group of adult subjects with normal and impaired renal function, as summarized in TABLE 8:

Total systemic clearance (CL) of foscarnet decreased and half-life increased with diminishing renal function (as expressed by creatinine clearance). Based on these observations, it is necessary to modify the dosage of foscarnet in patients with renal impairment.
Drug Interaction
- The pharmacokinetics of foscarnet and ganciclovir were not altered in 13 patients receiving either concomitant therapy or daily alternating therapy for maintenance of CMV disease.
- There is no clinically significant interaction with zidovudine (AZT), or probenecid.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity studies were conducted in rats and mice at oral doses of 500 mg/kg/day and 250 mg/kg/day. Oral bioavailability in unfasted rodents is < 20%. No evidence of oncogenicity was reported at plasma drug levels equal to 1/3 and 1/5, respectively, of those in humans (at the maximum recommended human daily dose) as measured by the area-under-the-time/concentration curve (AUC).
- Foscarnet sodium showed genotoxic effects in the BALB/3T3 in vitro transformation assay at concentrations greater than 0.5 mcg/mL and an increased frequency of chromosome aberrations in the sister chromatid exchange assay at 1000 mcg/mL. A high dose of foscarnet (350 mg/kg) caused an increase in micronucleated polychromatic erythrocytes in vivo in mice at doses that produced exposures (area under curve) comparable to that anticipated clinically.
Clinical Studies
CMV Retinitis
- A prospective, randomized, controlled clinical trial (FOS-03) was conducted in 24 patients with AIDS and CMV retinitis comparing treatment with foscarnet sodium to no treatment. Patients received induction treatment of foscarnet sodium, 60 mg/kg every 8 hours for 3 weeks, followed by maintenance treatment with 90 mg/kg/day until retinitis progression (appearance of a new lesion or advancement of the border of a posterior lesion greater than 750 microns in diameter). All diagnoses and determinations of retinitis progression were made from masked reading of retinal photographs. The 13 patients randomized to treatment with foscarnet sodium had a significant delay in progression of CMV retinitis compared to untreated controls. Median times to retinitis progression from study entry were 93 days (range 21 – >364) and 22 days (range 7 – 42), respectively.
- In another prospective clinical trial of CMV retinitis in patients with AIDS (ACTG-915), 33 patients were treated with two to three weeks of foscarnet sodium induction (60 mg/kg TID) and then randomized to either 90 mg/kg/day or 120 mg/kg/day maintenance therapy. The median times from study entry to retinitis progression were not significantly different between the treatment groups, 96 (range 14 – >176) days and 140 (range 16 – >233) days, respectively.
- In study ACTG 129/FGCRT SOCA study 107 patients with newly diagnosed CMV retinitis were randomized to treatment with foscarnet sodium (induction: 60 mg/kg TID for 2 weeks; maintenance: 90 mg/kg QD) and 127 were randomized to treatment with ganciclovir (induction: 5 mg/kg BID; maintenance: 5 mg/kg QD). The median time to progression on the two drugs was similar (Fos=59 and Gcv=56 days).
Relapsed CMV Retinitis
- The CMV Retinitis Retreatment Trial (ACTG 228/SOCA CRRT) was a randomized, open-label comparison of foscarnet sodium or ganciclovir monotherapy to the combination of both drugs for the treatment of persistently active or relapsed CMV retinitis in patients with AIDS. Subjects were randomized to one of the three treatments: foscarnet sodium 90 mg/kg BID induction followed by 120 mg/kg QD maintenance (Fos); ganciclovir 5 mg/kg BID induction followed by 10 mg/kg QD maintenance (Gcv); or the combination of the two drugs, consisting of continuation of the subject's current therapy and induction dosing of the other drug (as above), followed by maintenance with foscarnet sodium 90 mg/kg QD plus ganciclovir 5 mg/kg QD (Cmb). Assessment of retinitis progression was performed by masked evaluation of retinal photographs. The median times to retinitis progression or death were 39 days for the foscarnet sodium group, 61 days for the ganciclovir group and 105 days for the combination group. For the alternative endpoint of retinitis progression (censoring on death), the median times were 39 days for the foscarnet sodium group, 61 days for the ganciclovir group and 132 days for the combination group. Due to censoring on death, the latter analysis may overestimate the treatment effect. Treatment modifications due to toxicity were more common in the combination group than in the foscarnet sodium or ganciclovir monotherapy groups.
Mucocutaneous Acyclovir Resistant HSV Infections
- In a controlled trial, patients with AIDS and mucocutaneous, acyclovir-resistant HSV infection were randomized to either foscarnet sodium (N=8) at a dose of 40 mg/kg TID or vidarabine (N=6) at a dose of 15 mg/kg per day. Eleven patients were nonrandomly assigned to receive treatment with foscarnet sodium because of prior intolerance to vidarabine. Lesions in the eight patients randomized to foscarnet sodium healed after 11 to 25 days; seven of the 11 patients nonrandomly treated with foscarnet sodium healed their lesions in 10 to 30 days. Vidarabine was discontinued because of intolerance (N=4) or poor therapeutic response (N=2). In a second trial, forty AIDS patients and three bone marrow transplant recipients with mucocutaneous, acyclovir-resistant HSV infections were randomized to receive foscarnet sodium at a dose of either 40 mg/kg BID or 40 mg/kg TID. Fifteen of the 43 patients had healing of their lesions in 11 to 72 days with no difference in response between the two treatment groups.
How Supplied
- foscarnet sodium (foscarnet sodium) INJECTION, 24 mg/mL for intravenous infusion, is supplied in 250 mL glass bottles containing 6000 mg foscarnet sodium (24 mg/mL) as follows:
- NDC 76310-024-01 250 mL bottles, cases of 10
- For Single Use Only.
Storage
- Store between 20° and 25°C (68° and 77°F) [See USP Controlled Room Temperature]. Protect from excessive heat (above 40°C) and from freezing. If refrigerated or exposed to temperatures below the freezing point, precipitation may occur. By keeping the bottle at room temperature with repeated shaking, the precipitate can be brought into solution again.
- foscarnet sodium injection should be used only if the bottle and seal are intact, a vacuum is present, and the solution is clear and colorless.
Images
Drug Images
{{#ask: Page Name::Foscarnet sodium |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Foscarnet sodium |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Information for Patients
- CMV Retinitis: Patients should be advised that foscarnet sodium is not a cure for CMV retinitis, and that they may continue to experience progression of retinitis during or following treatment. They should be advised to have regular ophthalmologic examinations.
- Mucocutaneous Acyclovir-Resistant HSV Infections: Patients should be advised that foscarnet sodium is not a cure for HSV infections. While complete healing is possible, relapse occurs in most patients. Because relapse may be due to acyclovir-sensitive HSV, sensitivity testing of the viral isolate is advised. In addition, repeated treatment with foscarnet sodium has led to the development of resistance associated with poorer response. In the case of poor therapeutic response, sensitivity testing of the viral isolate also is advised.
- Effects on Ability to Drive and Use Machines: Adverse effects such as dizziness and convulsions may occur during foscarnet sodium therapy. Patients who experience seizures, dizziness, somnolence or other adverse reactions that could result in impairment, should be advised to avoid driving or operating machinery.
- General: Patients should be informed that the major toxicities of foscarnet are renal impairment, electrolyte disturbances, and seizures, and that dose modifications and possibly discontinuation may be required. The importance of close monitoring while on therapy must be emphasized. Patients should be advised of the importance of reporting to their physicians symptoms of perioral tingling, numbness in the extremities or paresthesias during or after infusion as possible symptoms of electrolyte abnormalities. Should such symptoms occur, the infusion of FOSCAVIR should be stopped, appropriate laboratory samples for assessment of electrolyte concentrations obtained, and a physician consulted before resuming treatment. The rate of infusion must be no more than 1 mg/kg/minute. The potential for renal impairment may be minimized by accompanying FOSCAVIR administration with hydration adequate to establish and maintain a diuresis during dosing.
Precautions with Alcohol
- Alcohol-Foscarnet sodium interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- FOSCAVIR
Look-Alike Drug Names
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Foscarnet sodium
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Foscarnet sodium |Label Name=Foscavir image01.jpg
}}
{{#subobject:
|Label Page=Foscarnet sodium |Label Name=Foscavir image.jpg
}}
{{#subobject:
|Label Page=Foscarnet sodium |Label Name=Foscavir ingredients and appearance.png
}}