Fluticasone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Fluticasone is a corticosteroid that is FDA approved for the treatment of asthma, management of the nasal symptoms of seasonal and perennial allergic rhinitis and nonallergic rhinitis, inflammatory and pruritic manifestations of atopic dermatitis. Common adverse reactions include candidiasis of mouth and esophagus, nausea and vomiting, osteoporosis, headache, cough, epistaxis, pharyngitis, sinusitis, throat irritation, upper respiratory infection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Asthma
- Fluticasone should be administered by the orally inhaled route only in patients aged 4 years and older. Individual patients will experience a variable time to onset and degree of symptom relief. Maximum benefit may not be achieved for 1 to 2 weeks or longer after starting treatment.
- After asthma stability has been achieved, it is always desirable to titrate to the lowest effective dosage to reduce the possibility of side effects. For patients who do not respond adequately to the starting dosage after 2 weeks of therapy, higher dosages may provide additional asthma control. The safety and efficacy of fluticasone when administered in excess of recommended dosages have not been established.
- The recommended starting dosage and the highest recommended dosage of fluticasone, based on prior asthma therapy, are listed below:
- Bronchodilators alone: Initial dose of 88 mcg twice daily to a maximum of 440 mcg twice daily.
- Inhaled corticosteroids: Initial dose of 88-220 mcg twice dailya, to a maximum of 440 mcg twice daily.
- Oral corticosteroidsb: Initial dose of 440 mcg twice daily, to a maximum of 880 mcg twice daily.
a Starting dosages above 88 mcg twice daily may be considered for patients with poorer asthma control or those who have previously required doses of inhaled corticosteroids that are in the higher range for the specific agent. b For patients currently receiving chronic oral corticosteroid therapy, prednisone should be reduced no faster than 2.5 to 5 mg/day on a weekly basis beginning after at least 1 week of therapy with fluticasone. Patients should be carefully monitored for signs of asthma instability, including serial objective measures of airflow, and for signs of adrenal insufficiency. Once prednisone reduction is complete, the dosage of fluticasone should be reduced to the lowest effective dosage.
Fluticasone should be primed before using for the first time by releasing 4 test sprays into the air away from the face, shaking well for 5 seconds before each spray. In cases where the inhaler has not been used for more than 7 days or when it has been dropped, prime the inhaler again by shaking well for 5 seconds and releasing 1 test spray into the air away from the face.
Rhinitis
- Dosing Information
- The recommended starting dosage in adults is 2 sprays (50 mcg of fluticasone propionate each) in each nostril once daily (total daily dose, 200 mcg). The same dosage divided into 100 mcg given twice daily (e.g., 8 a.m. and 8 p.m.) is also effective. After the first few days, patients may be able to reduce their dosage to 100 mcg (1 spray in each nostril) once daily for maintenance therapy. Some patients (12 years of age and older) with seasonal allergic rhinitis may find as-needed use of 200 mcg once daily effective for symptom control. Greater symptom control may be achieved with scheduled regular use.
Atopic Dermatitis
- Dosing information
- Apply a thin film of fluticasone to the affected skin areas once daily. Rub in gently.
- As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, reassessment of diagnosis may be necessary. The safety and efficacy of drug use for longer than 4 weeks have not been established.
- Fluticasone should not be used with occlusive dressings or applied in the diaper area unless directed by a physician.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Fluticasone in adult patients.
Non–Guideline-Supported Use
Nasal Polyp
- Dosing Information
- 200 mcg twice daily.[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Asthma
- Dosing Information: Pediatric patients (aged 4-11 years)c
- The recommended starting dosage and the highest recommended dosage of fluticasone, based on prior asthma therapy: Initial dose of 88 mcg twice daily to a maximum of 88 mcg twice daily.
c Recommended pediatric dosage is 88 mcg twice daily regardless of prior therapy. A valved holding chamber and mask may be used to deliver fluticasone to young patients.
Fluticasone should be primed before using for the first time by releasing 4 test sprays into the air away from the face, shaking well for 5 seconds before each spray. In cases where the inhaler has not been used for more than 7 days or when it has been dropped, prime the inhaler again by shaking well for 5 seconds and releasing 1 test spray into the air away from the face.
Condition 2
- Dosing Information
- Patients should be started with 100 mcg (1 spray in each nostril once daily). Patients not adequately responding to 100 mcg may use 200 mcg (2 sprays in each nostril). Once adequate control is achieved, the dosage should be decreased to 100 mcg (1 spray in each nostril) daily.
- The maximum total daily dosage should not exceed 2 sprays in each nostril (200 mcg/day).
- Fluticasone nasal spray is not recommended for children under 4 years of age.
Atopic Dermatitis
- Dosing information
- Apply a thin film of fluticasone to the affected skin areas once daily. Rub in gently.
- As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, reassessment of diagnosis may be necessary. The safety and efficacy of drug use for longer than 4 weeks have not been established.
- Fluticasone should not be used with occlusive dressings or applied in the diaper area unless directed by a physician.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Fluticasone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Fluticasone in pediatric patients.
Contraindications
The use of fluticasone is contraindicated in the following conditions:
- Primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required.
- Hypersensitivity to any of the ingredients in the formulation.
Warnings
Aerosol
Local Effects
In clinical studies, the development of localized infections of the mouth and pharynx with Candida albicans has occurred in patients treated with fluticasone. When such an infection develops, it should be treated with appropriate local or systemic (i.e., oral antifungal) therapy while treatment with fluticasone continues, but at times therapy with fluticasone may need to be interrupted. Patients should rinse the mouth after inhalation of fluticasone.
Acute Asthma Episodes
Fluticasone is not to be regarded as a bronchodilator and is not indicated for rapid relief of bronchospasm. Patients should be instructed to contact their physicians immediately when episodes of asthma that are not responsive to bronchodilators occur during the course of treatment with fluticasone. During such episodes, patients may require therapy with oral corticosteroids.
Immunosuppression
Persons who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In such children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If a patient is exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If a patient is exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. If chickenpox develops, treatment with antiviral agents may be considered.
Because of the potential for worsening infections, inhaled corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infection of the respiratory tract; untreated systemic fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
Transferring Patients From Systemic Corticosteroid Therapy
Particular care is needed for patients who have been transferred from systemically active corticosteroids to inhaled corticosteroids because deaths due to adrenal insufficiency have occurred in patients with asthma during and after transfer from systemic corticosteroids to less systemically available inhaled corticosteroids. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic-pituitary-adrenal (HPA) function.
Patients requiring oral corticosteroids should be weaned slowly from systemic corticosteroid use after transferring to fluticasone. In a clinical trial of 168 patients, prednisone reduction was successfully accomplished by reducing the daily prednisone dose on a weekly basis following initiation of treatment with fluticasone. Successive reduction of prednisone dose was allowed only when lung function, symptoms, and as-needed short-acting beta-agonist use were better than or comparable to that seen before initiation of prednisone dose reduction. Lung function (forced expiratory volume in 1 second [FEV1] or morning peak expiratory flow [AM PEF]), beta-agonist use, and asthma symptoms should be carefully monitored during withdrawal of oral corticosteroids. In addition to monitoring asthma signs and symptoms, patients should be observed for signs and symptoms of adrenal insufficiency such as fatigue, lassitude, weakness, nausea and vomiting, and hypotension.
Patients who have been previously maintained on 20 mg or more per day of prednisone (or its equivalent) may be most susceptible, particularly when their systemic corticosteroids have been almost completely withdrawn. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery, or infection (particularly gastroenteritis) or other conditions associated with severe electrolyte loss. Although inhaled corticosteroids may provide control of asthma symptoms during these episodes, in recommended doses they supply less than normal physiological amounts of glucocorticoid (cortisol) systemically and do not provide the mineralocorticoid activity that is necessary for coping with these emergencies.
During periods of stress or a severe asthma attack, patients who have been withdrawn from systemic corticosteroids should be instructed to resume oral corticosteroids immediately and to contact their physicians for further instruction. These patients should also be instructed to carry a warning card indicating that they may need supplementary systemic corticosteroids during periods of stress or a severe asthma attack.
Transfer of patients from systemic corticosteroid therapy to fluticasone may unmask conditions previously suppressed by the systemic corticosteroid therapy (e.g., rhinitis, conjunctivitis, eczema, arthritis, eosinophilic conditions). Some patients may experience symptoms of systemically active corticosteroid withdrawal (e.g., joint and/or muscular pain, lassitude, and depression, despite maintenance or even improvement of respiratory function).
Hypercorticism and Adrenal Suppression
Fluticasone propionate will often help control asthma symptoms with less suppression of HPA function than therapeutically equivalent oral doses of prednisone. Since fluticasone propionate is absorbed into the circulation and can be systemically active at higher doses, the beneficial effects of fluticasone in minimizing HPA dysfunction may be expected only when recommended dosages are not exceeded and individual patients are titrated to the lowest effective dose. A relationship between plasma levels of fluticasone propionate and inhibitory effects on stimulated cortisol production has been shown after 4 weeks of treatment with fluticasone propionate. Since individual sensitivity to effects on cortisol production exists, physicians should consider this information when prescribing fluticasone.
Because of the possibility of systemic absorption of inhaled corticosteroids, patients treated with fluticasone should be observed carefully for any evidence of systemic corticosteroid effects. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response.
It is possible that systemic corticosteroid effects such as hypercorticism and adrenal suppression (including adrenal crisis) may appear in a small number of patients, particularly when fluticasone is administered at higher than recommended doses over prolonged periods of time. If such effects occur, the dosage of fluticasone should be reduced slowly, consistent with accepted procedures for reducing systemic corticosteroids and for management of asthma.
Hypersensitivity Reactions, Including Anaphylaxis
Hypersensitivity reactions, including anaphylaxis, angioedema, urticaria, and bronchospasm, may occur after administration of fluticasone.
Reduction in Bone Mineral Density
Decreases in bone mineral density (BMD) have been observed with long-term administration of products containing inhaled corticosteroids. The clinical significance of small changes in BMD with regard to long-term outcomes is unknown. Patients with major risk factors for decreased bone mineral content, such as prolonged immobilization, family history of osteoporosis, postmenopausal status, tobacco use, advanced age, poor nutrition, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants, oral corticosteroids), should be monitored and treated with established standards of care.
Effect on Growth
Orally inhaled corticosteroids may cause a reduction in growth velocity when administered to pediatric patients. Monitor the growth of pediatric patients receiving fluticasone routinely (e.g., via stadiometry). To minimize the systemic effects of orally inhaled corticosteroids, including fluticasone, titrate each patient’s dosage to the lowest dosage that effectively controls his/her symptoms.
Glaucoma and Cataracts
Glaucoma, increased intraocular pressure, and cataracts have been reported in patients following the long-term administration of inhaled corticosteroids, including fluticasone propionate. Therefore, close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts.
Paradoxical Bronchospasm
As with other inhaled medications, bronchospasm may occur with an immediate increase in wheezing after dosing. If bronchospasm occurs following dosing with fluticasone, it should be treated immediately with a fast-acting inhaled bronchodilator. Treatment with fluticasone should be discontinued immediately and alternative therapy instituted.
Drug Interactions With Strong Cytochrome P450 3A4 Inhibitors
The use of strong cytochrome P450 3A4 (CYP3A4) inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, ketoconazole, telithromycin) with fluticasone is not recommended because increased systemic corticosteroid adverse effects may occur.
Eosinophilic Conditions and Churg-Strauss Syndrome
In rare cases, patients on inhaled fluticasone propionate may present with systemic eosinophilic conditions. Some of these patients have clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition that is often treated with systemic corticosteroid therapy. These events usually, but not always, have been associated with the reduction and/or withdrawal of oral corticosteroid therapy following the introduction of fluticasone propionate. Cases of serious eosinophilic conditions have also been reported with other inhaled corticosteroid in this clinical setting. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal relationship between fluticasone propionate and these underlying conditions has not been established.
Spray
- The replacement of a systemic corticosteroids with a topical corticosteroids can be accompanied by signs of adrenal insufficiency, and in addition some patients may experience symptoms of withdrawal, e.g., joint and/or muscular pain, lassitude, and depression. Patients previously treated for prolonged periods with systemic corticosteroids and transferred to topical corticosteroids should be carefully monitored for acute adrenal insufficiency in response to stress. In those patients who have asthma or other clinical conditions requiring long-term systemic corticosteroids treatment, too rapid a decrease in systemic corticosteroids may cause a severe exacerbation of their symptoms.
- The concomitant use of intranasal corticosteroids with other inhaled corticosteroids could increase the risk of signs or symptoms of hypercorticism and/or suppression of the HPA axis.
- A drug interaction study in healthy subjects has shown that ritonavir (a highly potent cytochrome P450 3A4 inhibitor) can significantly increase plasma fluticasone propionate exposure, resulting in significantly reduced serum cortisol concentrations.
- Persons who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
- Avoid spraying in eyes.
Precautions
Spray
- Intranasal corticosteroids may cause a reduction in growth velocity when administered to pediatric patients.
- Rarely, immediate hypersensitivity reactions or contact dermatitis may occur after the administration of fluticasone nasal spray. Rare instances of wheezing, nasal septum perforation, cataracts, glaucoma, and increased intraocular pressure have been reported following the intranasal application of corticosteroids, including fluticasone propionate.
- Use of excessive doses of corticosteroids may lead to signs or symptoms of hypercorticism and/or suppression of HPA function.
- Although systemic effects have been minimal with recommended doses of fluticasone nasal spray, potential risk increases with larger doses. Therefore, larger than recommended doses of fluticasone nasal spray should be avoided.
- When used at higher than recommended doses or in rare individuals at recommended doses, systemic corticosteroid effects such as hypercorticism and adrenal suppression may appear. If such changes occur, the dosage of fluticasone nasal spray should be discontinued slowly consistent with accepted procedures for discontinuing oral corticosteroid therapy.
- In clinical studies with fluticasone propionate administered intranasally, the development of localized infections of the nose and pharynx with Candida albicans has occurred only rarely. When such an infection develops, it may require treatment with appropriate local therapy and discontinuation of treatment with fluticasone nasal spray. Patients using fluticasone nasal spray over several months or longer should be examined periodically for evidence of Candida infection or other signs of adverse effects on the nasal mucosa.
- Intranasal corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculous infections of the respiratory tract; untreated local or systemic fungal or bacterial infections; systemic viral or parasitic infections; or ocular herpes simplex.
- Because of the inhibitory effect of corticosteroids on wound healing, patients who have experienced recent nasal septal ulcers, nasal surgery, or nasal trauma should not use a nasal corticosteroid until healing has occurred.
Lotion/Cream
- Fluticasone contains the excipient imidurea which releases formaldehyde as a breakdown product. Formaldehyde may cause allergic sensitization or irritation upon contact with the skin. Fluticasone should not be used in individuals with hypersensitivity to formaldehyde as it may prevent healing or worsen dermatitis.
- Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal axis (HPA) suppression with the potential for glucocorticosteroid insufficiency after withdrawal from treatment. Manifestations of Cushing's syndrome, hyperglycemia, and glucosuria can also be produced in some patients by systemic absorption of topical corticosteroids while on treatment.
- Patients applying a potent topical steroid to a large surface area or to areas under occlusion should be evaluated periodically for evidence of HPA axis suppression. This may be done by using cosyntropin (ACTH1·24) stimulation testing.
- Forty-two pediatric patients (4 months to < 6 years of age) with moderate to severe atopic eczema who were treated with fluticasone for at least 3-4 weeks were assessed for HPA axis suppression and 40 of these subjects applied at least 90% of applications. None of the 40 evaluable patients suppressed, where the sole criterion for HPA axis suppression is a plasma cortisol level of less than or equal to 18 micrograms per deciliter after cosyntropin stimulation. Although HPA axis suppression was observed in 0 of 40 pediatric patients (upper 95% confidence bound is 7.2%), the occurrence of HPA axis suppression in any patient and especially with longer use cannot be ruled out. In other studies with fluticasone propionate topical formulations, adrenal suppression has been observed.
- If HPA axis suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid. Recovery of HPA axis function is generally prompt upon discontinuation of topical corticosteroids. Infrequently, signs and symptoms of glucocorticosteroid insufficiency may occur requiring supplemental systemic corticosteroids. For information on systemic supplementation, see prescribing information for those products.
- Pediatric patients may be more susceptible to systemic toxicity from equivalent doses due to their larger skin surface to body mass ratios.
- The following local adverse reactions have been reported with topical corticosteroids, and they may occur more frequently with the use of occlusive dressings and higher potency corticosteroids. These reactions are listed in an approximately decreasing order of occurrence: irritation, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, skin atrophy, striae, hypertrichosis, and miliaria.
- Fluticasone, 0.05% may cause local cutaneous adverse reactions.
- If irritation develops, fluticasone should be discontinued and appropriate therapy instituted. Allergic contact dermatitis with corticosteroids is usually diagnosed by observing failure to heal rather than noting a clinical exacerbation as with most topical products not containing corticosteroids. Such an observation should be corroborated with appropriate diagnostic patch testing.
- If concomitant skin infections are present or develop, an appropriate antifungal or antibacterial agent should be used. If a favorable response does not occur promptly, use of fluticasone should be discontinued until the infection has been adequately controlled.
- Fluticasone should not be used in the presence of preexisting skin atrophy and should not be used where infection is present at the treatment site. Fluticasone should not be used in the treatment of rosacea and perioral dermatitis.
- Patients that apply fluticasone to exposed portions of the body should avoid excessive exposure to either natural or artificial sunlight (including tanning booths, sun lamps, etc.).
Adverse Reactions
Clinical Trials Experience
Aerosol
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice. The incidence of common adverse reactions in the table below is based upon 2 placebo-controlled US clinical trials in which 812 adult and adolescent patients (457 females and 355 males) previously treated with as-needed bronchodilators and/or inhaled corticosteroids were treated twice daily for up to 12 weeks with 2 inhalations of fluticasone 44 mcg Inhalation Aerosol,fluticasone 110 mcg Inhalation Aerosol,fluticasone 220 mcg Inhalation Aerosol (dosages of 88, 220, or 440 mcg twice daily), or placebo.
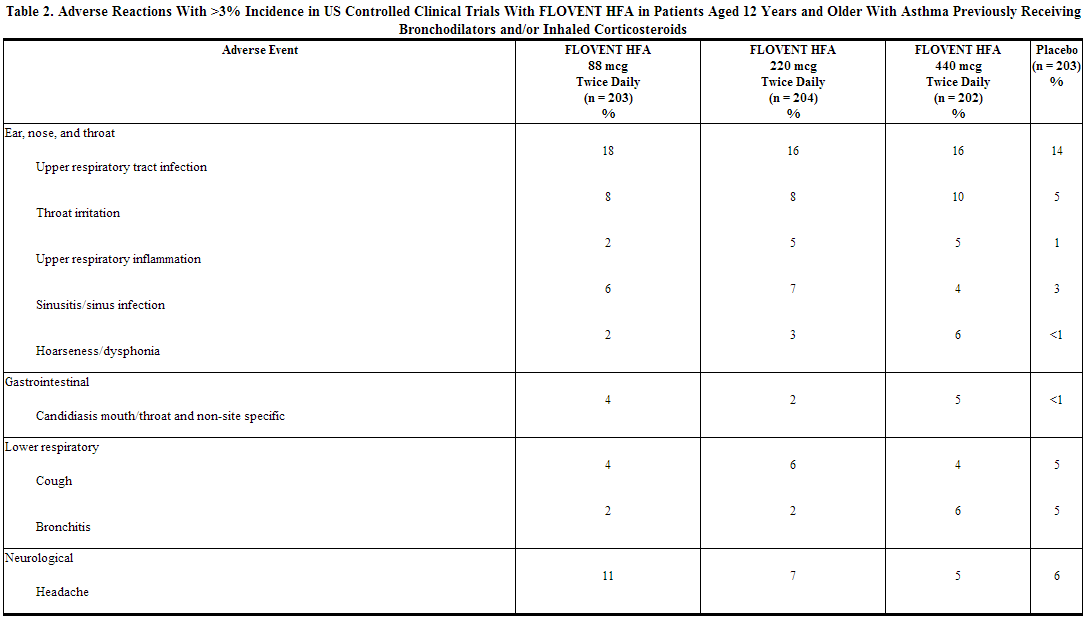
The table above includes all events (whether considered drug-related or nondrug-related by the investigator) that occurred at a rate of over 3% in any of the groups treated with fluticasone and were more common than in the placebo group. Less than 2% of patients discontinued from the studies because of adverse reactions. The average duration of exposure was 73 to 76 days in the active treatment groups compared with 60 days in the placebo group.
Additional Adverse Reactions: Other adverse reactions not previously listed, whether considered drug-related or not by the investigators, that were reported more frequently by patients with asthma treated with fluticasone compared with patients treated with placebo include the following: rhinitis, rhinorrhea/post-nasal drip, nasal sinus disorders, laryngitis, diarrhea, viral gastrointestinal infections, dyspeptic symptoms, gastrointestinal discomfort and pain, hyposalivation, musculoskeletal pain, muscle pain, muscle stiffness/tightness/rigidity, dizziness, migraines, fever, viral infections, pain, chest symptoms, viral skin infections, muscle injuries, soft tissue injuries, urinary infections.
Fluticasone propionate inhalation aerosol (440 or 880 mcg twice daily) was administered for 16 weeks to 168 patients with asthma requiring oral corticosteroids (Study 3). Adverse reactions not included above, but reported by more than 3 patients in either group treated with fluticasone and more commonly than in the placebo group included nausea and vomiting, arthralgia and articular rheumatism, and malaise and fatigue. In 2 long-term studies (26 and 52 weeks), the pattern of adverse reactions in patients treated with fluticasone at dosages up to 440 mcg twice daily was similar to that observed in the 12-week studies. There were no new and/or unexpected adverse reactions with long-term treatment. Pediatric Patients Aged 4 to 11 Years: fluticasone has been evaluated for safety in 56 pediatric patients who received 88 mcg twice daily for 4 weeks. Types of adverse reactions in these pediatric patients were generally similar to those observed in adults and adolescents.
Spray
In controlled US studies, more than 3,300 patients with seasonal allergic, perennial allergic, or perennial nonallergic rhinitis received treatment with intranasal fluticasone propionate. In general, adverse reactions in clinical studies have been primarily associated with irritation of the nasal mucous membranes, and the adverse reactions were reported with approximately the same frequency by patients treated with the vehicle itself. The complaints did not usually interfere with treatment. Less than 2% of patients in clinical trials discontinued because of adverse events; this rate was similar for vehicle placebo and active comparators.
Systemic corticosteroid side effects were not reported during controlled clinical studies up to 6 months’ duration with fluticasone nasal spray. If recommended doses are exceeded, however, or if individuals are particularly sensitive or taking fluticasone nasal spray in conjunction with administration of other corticosteroids, symptoms of hypercorticism, e.g., Cushing syndrome, could occur.
The following incidence of common adverse reactions (>3%, where incidence in fluticasone propionate-treated subjects exceeded placebo) is based upon 7 controlled clinical trials in which 536 patients (57 girls and 108 boys aged 4 to 11 years, 137 female and 234 male adolescents and adults) were treated with fluticasone nasal spray 200 mcg once daily over 2 to 4 weeks and 2 controlled clinical trials in which 246 patients (119 female and 127 male adolescents and adults) were treated with fluticasone nasal spray 200 mcg once daily over 6 months. Also included in the table are adverse events from 2 studies in which 167 children (45 girls and 122 boys aged 4 to 11 years) were treated with fluticasone nasal spray 100 mcg once daily for 2 to 4 weeks.
Overall Adverse Experiences With >3% Incidence on Fluticasone Propionate in Controlled Clinical Trials With fluticasone nasal spray in Patients ≥4 Years With Seasonal or Perennial Allergic Rhinitis.
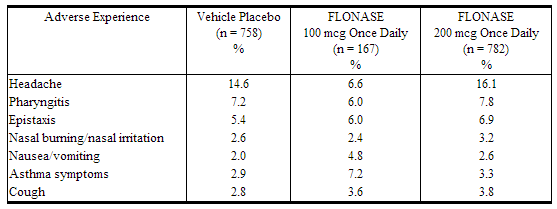
Other adverse events that occurred in ≤3% but ≥1% of patients and that were more common with fluticasone propionate (with uncertain relationship to treatment) included: blood in nasal mucus, runny nose, abdominal pain, diarrhea, fever, flu-like symptoms, aches and pains, dizziness, bronchitis.
Observed During Clinical Practice
In addition to adverse events reported from clinical trials, the following events have been identified during postapproval use of intranasal fluticasone propionate in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to fluticasone propionate or a combination of these factors.
- General: Hypersensitivity reactions, including angioedema, skin rash, edema of the face and tongue, pruritus, urticaria, bronchospasm, wheezing, dyspnea, and anaphylaxis/anaphylactoid reactions, which in rare instances were severe.
- Ear, Nose, and Throat: Alteration or loss of sense of taste and/or smell and, rarely, nasal septal perforation, nasal ulcer, sore throat, throat irritation and dryness, cough, hoarseness, and voice changes.
- Eye: Dryness and irritation, conjunctivitis, blurred vision, glaucoma, increased intraocular pressure, and cataracts.
- Cases of growth suppression have been reported for intranasal corticosteroids, including fluticasone.
Cream/Lotion
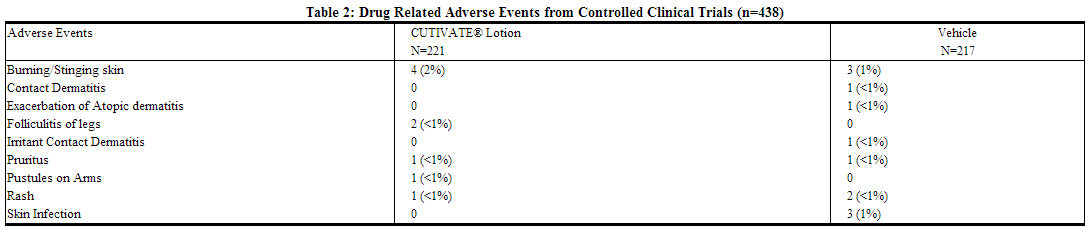
In 2 multicenter vehicle-controlled clinical trials of once-daily application of fluticasone by 196 adult and 242 pediatric patients, the total incidence of adverse reactions considered drug related by investigators was approximately 4%. Events were local cutaneous events, usually mild and self-limiting, and consisted primarily of burning/stinging (2%). All other drug-related events occurred with an incidence of less than 1%, and inclusively were contact dermatitis, exacerbation of atopic dermatitis, folliculitis of legs, pruritus, pustules on arm, rash, and skin infection. The incidence of drug-related events on drug compared to vehicle (4% and 5%, respectively) was similar. The incidence of drug-related events between study populations of 242 pediatric patients (age 3 months to < 17 years) and 196 adult patients (17 years or older) (4% and 5%, respectively) was also similar.
In an open-label study of 44 pediatric patients applying fluticasone to at least 35% of body surface area twice daily for 3 or 4 weeks, the overall incidence of drug-related adverse events was 14%. Events were local, cutaneous, and inclusively were dry skin (7%), stinging at application site (5%), and excoriation (2%).
The table below summarizes all adverse events by body system that occurred in at least 1% of patients in either the drug or vehicle group in controlled clinical trials.

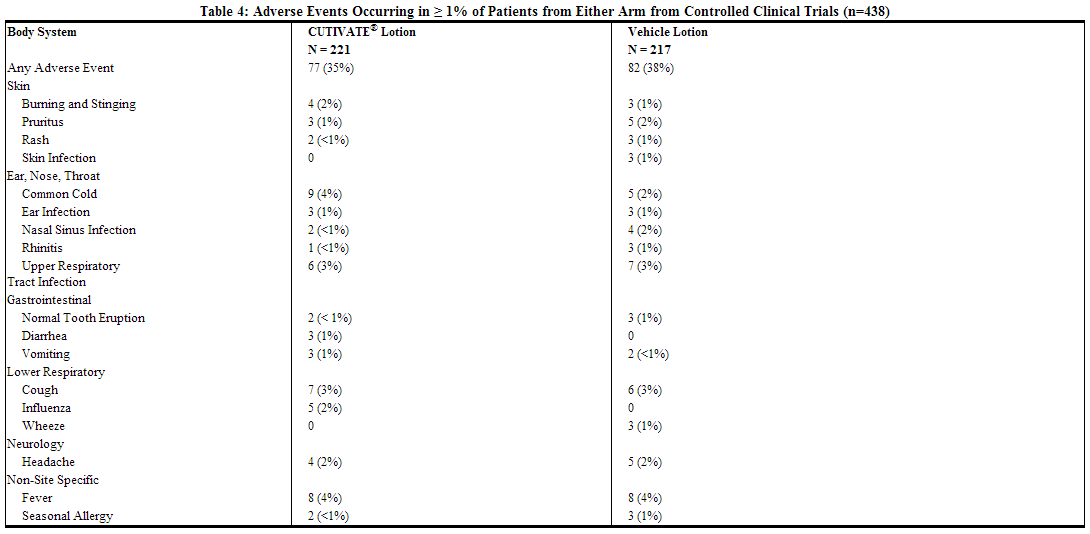
During the clinical trials, eczema herpeticum occurred in a 33-year-old male patient treated with fluticasone. Additionally, a 4-month-old patient treated with fluticasone in the open-label trial had marked elevations of the hepatic enzymes AST and ALT.
Postmarketing Experience
Aerosol
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been identified during postmarketing use of fluticasone propionate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to fluticasone propionate or a combination of these factors.
- Ear, Nose, and Throat: Aphonia, facial and oropharyngeal edema, and throat soreness and irritation.
- Endocrine and Metabolic: Cushingoid features, growth velocity reduction in children/adolescents, hyperglycemia, osteoporosis, and weight gain.
- Eye: Cataracts.
- Gastrointestinal Disorders: Dental caries and tooth discoloration.
- Immune System Disorders: Immediate and delayed hypersensitivity reactions, including urticaria, anaphylaxis, rash, and angioedema and bronchospasm, have been reported.
- Infections and Infestations: Esophageal candidiasis.
- Psychiatry: Agitation, aggression, anxiety, depression, and restlessness. Behavioral changes, including hyperactivity and irritability, have been reported very rarely and primarily in children.
- Respiratory: Asthma exacerbation, chest tightness, cough, dyspnea, immediate and delayed bronchospasm, paradoxical bronchospasm, pneumonia, and wheeze.
- Skin: Contusions, cutaneous hypersensitivity reactions, ecchymoses, and pruritus.
- Eosinophilic Conditions: In rare cases, patients on inhaled fluticasone propionate may present with systemic eosinophilic conditions, with some patients presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition that is often treated with systemic corticosteroid therapy. These events usually, but not always, have been associated with the reduction and/or withdrawal of oral corticosteroid therapy following the introduction of fluticasone propionate
Spray
During postmarketing use, there have been reports of clinically significant drug interactions in patients receiving fluticasone propionate and ritonavir, resulting in systemic corticosteroid effects including Cushing syndrome and adrenal suppression. Therefore, coadministration of fluticasone propionate and ritonavir is not recommended unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects.
Lotion/Cream
Systemic adverse events with fluticasone cream and fluticasone ointment have included:
- Immunosuppression/Pneumocystis carinii pneumonia/leukopenia/thrombocytopenia
- Hyperglycemia/ glycosuria
- Cushing syndrome
- Generalized body edema/blurred vision
- Acute urticarial reaction (edema, urticaria, pruritus, and throat swelling)
The following localized adverse reactions have been reported during post approval use of fluticasone:
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Drug Interactions
Strong Cytochrome P450 3A4 Inhibitors
- Fluticasone propionate is a substrate of CYP3A4. The use of strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, ketoconazole, telithromycin) with fluticasone is not recommended because increased systemic corticosteroid adverse effects may occur.
- A drug interaction study with fluticasone propionate aqueous nasal spray in healthy subjects has shown that ritonavir (a strong CYP3A4 inhibitor) can significantly increase plasma fluticasone propionate concentration, resulting in significantly reduced serum cortisol concentrations. During postmarketing use, there have been reports of clinically significant drug interactions in patients receiving fluticasone propionate and ritonavir, resulting in systemic corticosteroid effects including Cushing’s syndrome and adrenal suppression. Therefore, coadministration of fluticasone propionate and ritonavir is not recommended unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects.
- Coadministration of orally inhaled fluticasone propionate (1,000 mcg) and ketoconazole (200 mg once daily) resulted in a 1.9-fold increase in plasma fluticasone propionate exposure and a 45% decrease in plasma cortisol area under the curve (AUC), but had no effect on urinary excretion of cortisol. Coadministration of fluticasone propionate and ketoconazole is not recommended unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects.
Erythromycin
- In a multiple-dose drug interaction study, coadministration of orally inhaled fluticasone propionate (500 mcg twice daily) and erythromycin (333 mg 3 times daily) did not affect fluticasone propionate pharmacokinetics.
Use in Specific Populations
Pregnancy
Aerosol
There are no adequate and well-controlled studies with fluticasone in pregnant women. Fluticasone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Teratogenic Effects
Subcutaneous studies in mice at a dose approximately 0.1 times the maximum recommended human daily inhalation dose (MRHD) for adults on a mg/m2 basis and in the rat at a dose approximately 0.5 times the MRHD in adults on a mg/m2 basis revealed fetal toxicity characteristic of potent corticosteroid compounds, including embryonic growth retardation, omphalocele, cleft palate, and retarded cranial ossification.
In rabbits, fetal weight reduction and cleft palate were observed at a subcutaneous dose approximately 0.04 times the MRHD for adults on a mg/m2 basis. However, no teratogenic effects were reported at oral doses up to approximately 3 times the MRHD for adults on a mg/m2 basis. No fluticasone propionate was detected in the plasma in this study, consistent with the established low bioavailability following oral administration.
Experience with oral corticosteroids since their introduction in pharmacologic, as opposed to physiologic, doses suggests that rodents are more prone to teratogenic effects from corticosteroids than humans. In addition, because there is a natural increase in corticosteroid production during pregnancy, most women will require a lower exogenous corticosteroid dose and many will not need corticosteroid treatment during pregnancy.
Spray
Subcutaneous studies in the mouse and rat at 45 and 100 mcg/kg, respectively (approximately equivalent to and 4 times, respectively, the maximum recommended daily intranasal dose in adults on a mcg/m2 basis), revealed fetal toxicity characteristic of potent corticosteroid compounds, including embryonic growth retardation, omphalocele, cleft palate, and retarded cranial ossification.
In the rabbit, fetal weight reduction and cleft palate were observed at a subcutaneous dose of 4 mcg/kg (less than the maximum recommended daily intranasal dose in adults on a mcg/m2 basis). However, no teratogenic effects were reported at oral doses up to 300 mcg/kg (approximately 25 times the maximum recommended daily intranasal dose in adults on a mcg/m2 basis) of fluticasone propionate to the rabbit. No fluticasone propionate was detected in the plasma in this study, consistent with the established low bioavailability following oral administration.
Fluticasone propionate crossed the placenta following oral administration of 100 mcg/kg to rats and 300 mcg/kg to rabbits (approximately 4 and 25 times, respectively, the maximum recommended daily intranasal dose in adults on a mcg/m2 basis).
There are no adequate and well-controlled studies in pregnant women. Fluticasone propionate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Experience with oral corticosteroids since their introduction in pharmacologic, as opposed to physiologic, doses suggests that rodents are more prone to teratogenic effects from corticosteroids than humans. In addition, because there is a natural increase in corticosteroid production during pregnancy, most women will require a lower exogenous corticosteroid dose and many will not need corticosteroid treatment during pregnancy.
Lotion/Cream
Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
Systemic embryofetal development studies were conducted in mice, rats and rabbits. Subcutaneous doses of 15, 45 and 150 μg/kg/day of fluticasone propionate were administered to pregnant female mice from gestation days 6 – 15. A teratogenic effect characteristic of corticosteroids (cleft palate) was noted after administration of 45 and 150 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 15 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Subcutaneous doses of 10, 30 and 100 μg/kg/day of fluticasone propionate were administered to pregnant female rats in two embryofetal development studies (one study administered fluticasone propionate from gestation days 6 – 15 and the other study from gestation days 7 – 17). In the presence of maternal toxicity, fetal effects noted at 100 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) included decreased fetal weights, omphalocele, cleft palate, and retarded skeletal ossification. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 10 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Subcutaneous doses of 0.08, 0.57 and 4 μg/kg/day of fluticasone propionate were administered to pregnant female rabbits from gestation days 6 – 18. Fetal effects noted at 4 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) included decreased fetal weights, cleft palate and retarded skeletal ossification. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 0.57 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Oral doses of 3, 30 and 300 μg/kg/day fluticasone propionate were administered to pregnant female rabbits from gestation days 8 – 20. No fetal or teratogenic effects were noted at oral doses up to 300 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study. However, no fluticasone propionate was detected in the plasma in this study, consistent with the established low bioavailability following oral administration.
Fluticasone propionate crossed the placenta following administration of a subcutaneous or an oral dose of 100 μg/kg tritiated fluticasone propionate to pregnant rats.
There are no adequate and well-controlled studies in pregnant women. During clinical trials of fluticasone, women of childbearing potential were required to use contraception to avoid pregnancy. Therefore, fluticasone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Fluticasone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Fluticasone during labor and delivery.
Nursing Mothers
Aerosol
It is not known whether fluticasone propionate is excreted in human breast milk. However, other corticosteroids have been detected in human milk. Subcutaneous administration to lactating rats of tritiated fluticasone propionate (approximately 0.05 times the MRHD in adults on a mg/m2 basis) resulted in measurable radioactivity in milk.
Since there are no data from controlled trials on the use of fluticasone by nursing mothers, caution should be exercised when fluticasone is administered to a nursing woman.
Spray
Subcutaneous administration to lactating rats of 10 mcg/kg of tritiated fluticasone propionate (less than the maximum recommended daily intranasal dose in adults on a mcg/m2 basis) resulted in measurable radioactivity in the milk. Since there are no data from controlled trials on the use of intranasal fluticasone propionate by nursing mothers, caution should be exercised when fluticasone nasal spray is administered to a nursing woman.
Lotion/Cream
It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when fluticasone is administered to a nursing woman.
Pediatric Use
Aerosol
The safety and effectiveness of fluticasone in children aged 4 years and older have been established. The safety and effectiveness of fluticasone in children younger than 4 years have not been established. Use of fluticasone in patients aged 4 to 11 years is supported by evidence from adequate and well-controlled studies in adults and adolescents aged 12 years and older, pharmacokinetic studies in patients aged 4 to 11 years, established efficacy of fluticasone propionate formulated as fluticasone inhalation powder and fluticasone inhalation powder in patients aged 4 to 11 years, and supportive findings with fluticasone in a study conducted in patients aged 4 to 11 years.
Effects on Growth
Orally inhaled corticosteroids may cause a reduction in growth velocity when administered to pediatric patients. A reduction of growth velocity in children or teenagers may occur as a result of poorly controlled asthma or from use of corticosteroids including inhaled corticosteroids. The effects of long-term treatment of children and adolescents with inhaled corticosteroids, including fluticasone propionate, on final adult height are not known.
Controlled clinical studies have shown that inhaled corticosteroids may cause a reduction in growth in pediatric patients. In these studies, the mean reduction in growth velocity was approximately 1 cm/year (range: 0.3 to 1.8 cm/year) and appeared to depend upon dose and duration of exposure. This effect was observed in the absence of laboratory evidence of HPA axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The long-term effects of this reduction in growth velocity associated with orally inhaled corticosteroids, including the impact on final adult height, are unknown. The potential for “catch-up” growth following discontinuation of treatment with orally inhaled corticosteroids has not been adequately studied. The effects on growth velocity of treatment with orally inhaled corticosteroids for over 1 year, including the impact on final adult height, are unknown. The growth of children and adolescents receiving orally inhaled corticosteroids, including fluticasone, should be monitored routinely (e.g., via stadiometry). The potential growth effects of prolonged treatment should be weighed against the clinical benefits obtained and the risks associated with alternative therapies. To minimize the systemic effects of orally inhaled corticosteroids, including fluticasone, each patient should be titrated to the lowest dose that effectively controls his/her symptoms.
Since a cross study comparison in adult and adolescent patients (aged 12 years and older) indicated that systemic exposure of inhaled fluticasone would be higher than exposure from fluticasone propionate inhalation powder, results from a study to assess the potential growth effects of fluticasone propionate inhalation powder in pediatric patients (aged 4 to 11 years) are provided.
A 52-week placebo-controlled study to assess the potential growth effects of fluticasone propionate inhalation powder at 50 and 100 mcg twice daily was conducted in the US in 325 prepubescent children (244 males and 81 females) aged 4 to 11 years. The mean growth velocities at 52 weeks observed in the intent-to-treat population were 6.32 cm/year in the placebo group (n = 76), 6.07 cm/year in the 50-mcg group (n = 98), and 5.66 cm/year in the 100-mcg group (n = 89). An imbalance in the proportion of children entering puberty between groups and a higher dropout rate in the placebo group due to poorly controlled asthma may be confounding factors in interpreting these data. A separate subset analysis of children who remained prepubertal during the study revealed growth rates at 52 weeks of 6.10 cm/year in the placebo group (n = 57), 5.91 cm/year in the 50-mcg group (n = 74), and 5.67 cm/year in the 100-mcg group (n = 79). In children aged 8.5 years, the mean age of children in this study, the range for expected growth velocity is: boys – 3rd percentile = 3.8 cm/year, 50th percentile = 5.4 cm/year, and 97th percentile = 7.0 cm/year; girls – 3rd percentile = 4.2 cm/year, 50th percentile = 5.7 cm/year, and 97th percentile = 7.3 cm/year. The clinical significance of these growth data is not certain. Physicians should closely follow the growth of children and adolescents taking corticosteroids by any route, and weigh the benefits of corticosteroid therapy against the possibility of growth suppression if growth appears slowed. Patients should be maintained on the lowest dose of inhaled corticosteroid that effectively controls their asthma.
Children Younger Than 4 Years:Pharmacokinetics: Pharmacodynamics: A 12-week, double-blind, placebo-controlled, parallel-group study was conducted in children with asthma aged 1 to younger than 4 years. Twelve-hour overnight urinary cortisol excretion after a 12-week treatment period with 88 mcg of fluticasone twice daily (n = 73) and with placebo (n = 42) were calculated. The mean and median change from baseline in urine cortisol over 12 hours were -0.7 and 0.0 mcg for fluticasone and 0.3 and -0.2 mcg for placebo, respectively.
In a 1-way crossover study in children aged 6 to younger than 12 months with reactive airways disease (N = 21), serum cortisol was measured over a 12-hour dosing period. Patients received placebo treatment for a 2-week period followed by a 4-week treatment period with 88 mcg of fluticasone twice daily with a valved holding Chamber (VHC) with mask. The geometric mean ratio of serum cortisol over 12 hours (AUC0-12 h) following fluticasone (n = 16) versus placebo (n = 18) was 0.95 (95% CI: 0.72, 1.27).
Safety: Fluticasone administered as 88 mcg twice daily has been evaluated for safety in 239 pediatric patients aged 1 to younger than 4 years in a 12-week, double-blind, placebo-controlled study. Treatments were administered with an valved holding Chamber (VHC) with mask. In pediatric patients aged 1 to younger than 4 years receiving fluticasone, the following events occurred with a frequency greater than 3% and more frequently than in pediatric patients who received placebo, regardless of causality assessment: pyrexia, nasopharyngitis, upper respiratory tract infection, vomiting, otitis media, diarrhea, bronchitis, pharyngitis, and viral infection.
Fluticasone administered as 88 mcg twice daily has also been evaluated for safety in 23 pediatric patients aged 6 to 12 months in an open-label placebo-controlled study. Treatments were administered with an valved holding Chamber (VHC) with mask for 2 weeks with placebo followed by 4 weeks with active drug. There was no discernable difference in the types of adverse events reported between patients receiving placebo compared to the active drug.
In Vitro Testing of Dose Delivery With Holding Chambers: In vitro dose characterization studies were performed to evaluate the delivery of fluticasone via holding chambers with attached masks. The studies were conducted with 2 different holding chambers (valved holding Chamber (VHC) and AeroChamber Z-STAT Plus™ VHC) with masks (small and medium size) at inspiratory flow rates of 4.9, 8.0, and 12.0 L/min in combination with holding times of 0, 2, 5, and 10 seconds. The flow rates were selected to be representative of inspiratory flow rates of children aged 6 to 12 months, 2 to 5 years, and over 5 years, respectively. The mean delivered dose of fluticasone propionate through the holding chambers with masks was lower than the 44 mcg of fluticasone propionate delivered directly from the actuator mouthpiece. The results were similar through both holding chambers. The fine particle fraction (approximately 1 to 5 μm) across the flow rates used in these studies was 70% to 84% of the delivered dose, consistent with the removal of the coarser fraction by the holding chamber. In contrast, the fine particle fraction for fluticasone delivered without a holding chamber typically represents 42% to 55% of the delivered dose measured at the standard flow rate of 28.3 L/min. These data suggest that, on a per kilogram basis, young children receive a comparable dose of fluticasone propionate when delivered via a holding chamber and mask as adults do without their use.
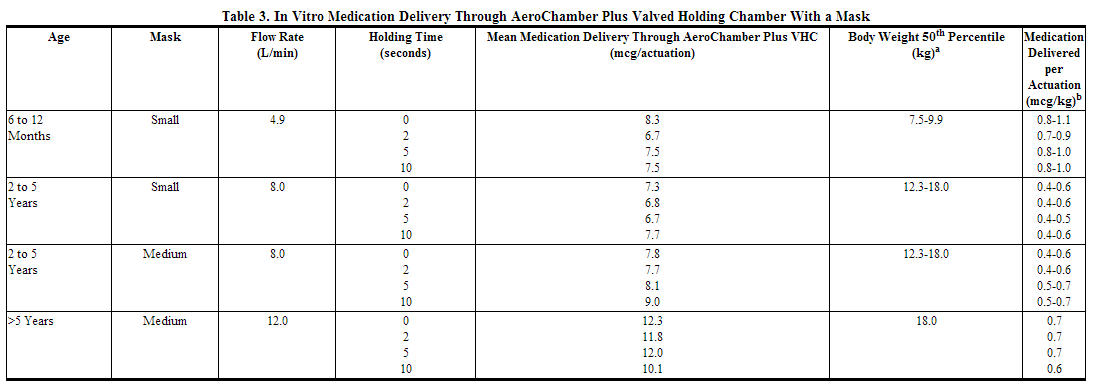
aCenters for Disease Control growth charts, developed by the National Center for Health Statistics in collaboration with the National Center for Chronic Disease Prevention and Health Promotion (2000). Ranges correspond to the average of the 50th percentile weight for boys and girls at the ages indicated. bA single inhalation of fluticasone in a 70-kg adult without use of a valved holding chamber and mask delivers approximately 44 mcg, or 0.6 mcg/kg.
Spray
Six hundred fifty (650) patients aged 4 to 11 years and 440 patients aged 12 to 17 years were studied in US clinical trials with fluticasone propionate nasal spray. The safety and effectiveness of fluticasone nasal spray in children below 4 years of age have not been established.
Controlled clinical studies have shown that intranasal corticosteroids may cause a reduction in growth velocity in pediatric patients. This effect has been observed in the absence of laboratory evidence of HPA axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The long-term effects of this reduction in growth velocity associated with intranasal corticosteroids, including the impact on final adult height, are unknown. The potential for “catch-up” growth following discontinuation of treatment with intranasal corticosteroids has not been adequately studied. The growth of pediatric patients receiving intranasal corticosteroids, including fluticasone nasal spray, should be monitored routinely (e.g., via stadiometry). The potential growth effects of prolonged treatment should be weighed against the clinical benefits obtained and the risks/benefits of treatment alternatives. To minimize the systemic effects of intranasal corticosteroids, including fluticasone nasal spray, each patient should be titrated to the lowest dose that effectively controls his/her symptoms.
A 1-year placebo-controlled clinical growth study was conducted in 150 pediatric patients (ages 3 to 9 years) to assess the effect of fluticasone nasal spray (single daily dose of 200 mcg, the maximum approved dose) on growth velocity. From the primary population of 56 patients receiving fluticasone nasal spray and 52 receiving placebo, the point estimate for growth velocity with fluticasone nasal spray was 0.14 cm/year lower than that noted with placebo (95% confidence interval ranging from 0.54 cm/year lower than placebo to 0.27 cm/year higher than placebo). Thus, no statistically significant effect on growth was noted compared to placebo. No evidence of clinically relevant changes in HPA axis function or bone mineral density was observed as assessed by 12-hour urinary cortisol excretion and dual-energy x-ray absorptiometry, respectively.
The potential for fluticasone nasal spray to cause growth suppression in susceptible patients or when given at higher doses cannot be ruled out.
Lotion/Cream
Fluticasone contains the excipient imidurea which releases formaldehyde as a breakdown product. Formaldehyde may cause allergic sensitization or irritation upon contact with the skin. Fluticasone should not be used in individuals with hypersensitivity to formaldehyde as it may prevent healing or worsen dermatitis.
Fluticasone should be discontinued if control is achieved before 4 weeks. If no improvement is seen within 2 weeks, contact a physician. The safety of the use of fluticasone for longer than 4 weeks has not been established.
The safety and efficacy of fluticasone in pediatric patients below 1 year of age have not been established.
Parents of pediatric patients should be advised not to use this medication in the treatment of diaper dermatitis unless directed by the physician. Fluticasone should not be applied in the diaper areas as diapers or plastic pants may constitute occlusive dressing.
Forty-two pediatric patients (4 months to < 6 years of age) with moderate to severe atopic eczema who were treated with Fluticasone for at least 3-4 weeks were assessed for HPA axis suppression and 40 of these subjects applied at least 90% of applications. None of the 40 evaluable patients suppressed, where the sole criterion for HPA axis suppression is a plasma cortisol level of less than or equal to 18 micrograms per deciliter after cosyntropin stimulation. Although HPA axis suppression was observed in 0 of 40 pediatric patients (upper 95% confidence bound is 7.2%), the occurrence of HPA axis suppression in any patient and especially with longer use cannot be ruled out.
In other studies with fluticasone propionate topical formulations, adrenal suppression has been observed. Fluticasone cream, 0.05% caused HPA axis suppression in 2 of 43 pediatric patients, ages 2 and 5 years old, who were treated for 4 weeks covering at least 35% of the body surface area. Follow-up testing 12 days after treatment discontinuation, available for 1 of the 2 patients, demonstrated a normally responsive HPA axis.
HPA axis suppression, Cushing's syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in pediatric patients receiving topical corticosteroids. Manifestations of adrenal suppression in pediatric patients include low plasma cortisol levels to an absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema. Administration of topical corticosteroids to children should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of children.
In addition, local adverse events including cutaneous atrophy, striae, telangiectasia, and pigmentation change have been reported with topical use of corticosteroids in pediatric patients.
Geriatic Use
Aerosol
Of the total number of patients treated with fluticasone in US and non-US clinical trials, 173 were aged 65 years or older, 19 of which were 75 years or older. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Spray
A limited number of patients 65 years of age and older (n = 129) or 75 years of age and older (n = 11) have been treated with fluticasone nasal spray in US and non-US clinical trials. While the number of patients is too small to permit separate analysis of efficacy and safety, the adverse reactions reported in this population were similar to those reported by younger patients.
Lotion/Cream
A limited number of patients above 65 years of age have been treated with fluticasone in US and non-US clinical trials. Specifically only 8 patients above 65 years of age were treated with fluticasone in controlled clinical trials. The number of patients is too small to permit separate analyses of efficacy and safety.
Gender
No significant difference in clearance (CL/F) of fluticasone propionate was observed.
Race
There is no FDA guidance on the use of Fluticasone with respect to specific racial populations.
Renal Impairment
Formal pharmacokinetic studies using fluticasone have not been conducted in patients with renal impairment.
Hepatic Impairment
Formal pharmacokinetic studies using fluticasone have not been conducted in patients with hepatic impairment. Since fluticasone propionate is predominantly cleared by hepatic metabolism, impairment of liver function may lead to accumulation of fluticasone propionate in plasma. Therefore, patients with hepatic disease should be closely monitored.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Fluticasone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Fluticasone in patients who are immunocompromised.
Administration and Monitoring
Administration
- Inhaled
- Spray
- Cutaneous application
Monitoring
Transferring Patients From Systemic Corticosteroid Therapy
Patients previously treated for prolonged periods with systemic corticosteroids and transferred to topical corticosteroids should be carefully monitored for acute adrenal insufficiency in response to stress.
Aerosol
Lung function (forced expiratory volume in 1 second [FEV1] or morning peak expiratory flow [AM PEF]), beta-agonist use, and asthma symptoms should be carefully monitored during withdrawal of oral corticosteroids.
IV Compatibility
There is limited information regarding the compatibility of Fluticasone and IV administrations.
Overdosage
- Chronic overdosage may result in signs/symptoms of hypercorticism.
- Inhalation by healthy volunteers of a single dose of 1,760 or 3,520 mcg of fluticasone was well tolerated. Doses of 1,320 mcg administered to healthy human volunteers twice daily for 7 to 15 days were also well tolerated.
- Intranasal administration of 2 mg (10 times the recommended dose) of fluticasone propionate twice daily for 7 days to healthy human volunteers was well tolerated. Single oral doses up to 16 mg have been studied in human volunteers with no acute toxic effects reported.
- Repeat oral doses up to 80 mg daily for 10 days in healthy volunteers and repeat oral doses up to 20 mg daily for 42 days in patients were well tolerated. Adverse reactions were of mild or moderate severity, and incidences were similar in active and placebo treatment groups.
- No deaths were seen in mice given an oral dose of 1,000 mg/kg (approximately 2,300 and 11,000 times the MRHD for adults and children aged 4 to 11 years, respectively, on a mg/m2 basis). No deaths were seen in rats given an oral dose of 1,000 mg/kg (approximately 4,600 and 22,000 times the MRHD in adults and children aged 4 to 11 years, respectively, on a mg/m2 basis).
- The oral and subcutaneous median lethal doses in mice and rats were >1,000 mg/kg (>20,000 and >41,000 times, respectively, the maximum recommended daily intranasal dose in adults and >10,000 and >20,000 times, respectively, the maximum recommended daily intranasal dose in children on a mg/m2 basis).
- Topically applied fluticasone can be absorbed in sufficient amounts to produce systemic effects.
Pharmacology
Mechanism of Action
Aerosol
Fluticasone propionate is a synthetic trifluorinated corticosteroid with potent anti-inflammatory activity. In vitro assays using human lung cytosol preparations have established fluticasone propionate as a human glucocorticoid receptor agonist with an affinity 18 times greater than dexamethasone, almost twice that of beclomethasone‑17‑monopropionate (BMP), the active metabolite of beclomethasone dipropionate, and over 3 times that of budesonide. Data from the McKenzie vasoconstrictor assay in man are consistent with these results. The clinical significance of these findings is unknown.
Inflammation is an important component in the pathogenesis of asthma. Corticosteroids have been shown to inhibit multiple cell types (e.g., mast cells, eosinophils, basophils, lymphocytes, macrophages, neutrophils) and mediator production or secretion (e.g., histamine, eicosanoids, leukotrienes, cytokines) involved in the asthmatic response. These anti-inflammatory actions of corticosteroids contribute to their efficacy in asthma.
Though effective for the treatment of asthma, corticosteroids do not affect asthma symptoms immediately. Individual patients will experience a variable time to onset and degree of symptom relief. Maximum benefit may not be achieved for 1 to 2 weeks or longer after starting treatment. When corticosteroids are discontinued, asthma stability may persist for several days or longer.
Studies in patients with asthma have shown a favorable ratio between topical anti-inflammatory activity and systemic corticosteroid effects with recommended doses of orally inhaled fluticasone propionate.
This is explained by a combination of a relatively high local anti-inflammatory effect, negligible oral systemic bioavailability (less than 1%), and the minimal pharmacological activity of the only metabolite detected in man.
Nasal Spray
Fluticasone propionate is a synthetic trifluorinated corticosteroid with anti-inflammatory activity. In vitro dose response studies on a cloned human glucocorticoid receptor system involving binding and gene expression afforded 50% responses at 1.25 and 0.17 nM concentrations, respectively. Fluticasone propionate was 3-fold to 5-fold more potent than dexamethasone in these assays. Data from the McKenzie vasoconstrictor assay in man also support its potent glucocorticoid activity.
In preclinical studies, fluticasone propionate revealed progesterone-like activity similar to the natural hormone. However, the clinical significance of these findings in relation to the low plasma levels (see Pharmacokinetics) is not known.
The precise mechanism through which fluticasone propionate affects allergic rhinitis symptoms is not known. Corticosteroids have been shown to have a wide range of effects on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in inflammation. In 7 trials in adults, fluticasone nasal spray has decreased nasal mucosal eosinophils in 66% (35% for placebo) of patients and basophils in 39% (28% for placebo) of patients. The direct relationship of these findings to long-term symptom relief is not known.
Fluticasone nasal spray, like other corticosteroids, is an agent that does not have an immediate effect on allergic symptoms. A decrease in nasal symptoms has been noted in some patients 12 hours after initial treatment with fluticasone nasal spray. Maximum benefit may not be reached for several days. Similarly, when corticosteroids are discontinued, symptoms may not return for several days.
Lotion/Spray
The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
Structure
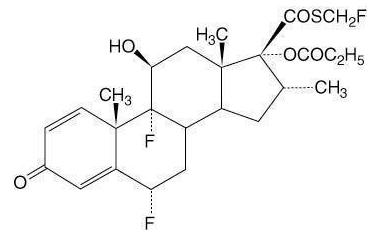
The active component of fluticaasone 44 mcg inhalation aerosol, fluticasone 110 mcg inhalation aerosol, and fluticasone 220 mcg inhalation aerosol is fluticasone propionate, a corticosteroid having the chemical name S-(fluoromethyl) 6α,9-difluoro-11β,17-dihydroxy-16α-methyl-3-oxoandrosta-1,4-diene-17β-carbothioate, 17-propionate and the following chemical structure:
Aerosol
- Fluticasone propionate is a white powder with a molecular weight of 500.6, and the empirical formula is C25H31F3O5S. It is practically insoluble in water, freely soluble in dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol.
- Fluticasone 44 mcg Inhalation Aerosol, fluticasone 110 mcg Inhalation Aerosol, and fluticasone 220 mcg inhalation aerosol are pressurized metered-dose aerosol units fitted with a counter. Fluticasone is intended for oral inhalation only. Each unit contains a microcrystalline suspension of fluticasone propionate (micronized) in propellant HFA-134a (1,1,1,2-tetrafluoroethane). It contains no other excipients.
- After priming, each actuation of the inhaler delivers 50, 125, or 250 mcg of fluticasone propionate in 60 mg of suspension (for the 44-mcg product) or in 75 mg of suspension (for the 110- and 220-mcg products) from the valve. Each actuation delivers 44, 110, or 220 mcg of fluticasone propionate from the actuator. The actual amount of drug delivered to the lung may depend on patient factors, such as the coordination between the actuation of the device and inspiration through the delivery system.
- Each 10.6-g canister (44 mcg) and each 12-g canister (110 and 220 mcg) provides 120 inhalations.
- Fluticasone should be primed before using for the first time by releasing 4 test sprays into the air away from the face, shaking well for 5 seconds before each spray. In cases where the inhaler has not been used for more than 7 days or when it has been dropped, prime the inhaler again by shaking well for 5 seconds and releasing 1 test spray into the air away from the face.
- This product does not contain any chlorofluorocarbon (CFC) as the propellant.
Nasal Spray
- Fluticasone propionate is a white powder with a molecular weight of 500.6, and the empirical formula is C25H31F3O5S. It is practically insoluble in water, freely soluble in dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol.
- Fluticasone nasal spray, 50 mcg is an aqueous suspension of microfine fluticasone propionate for topical administration to the nasal mucosa by means of a metering, atomizing spray pump. Fluticasone nasal spray also contains microcrystalline cellulose and carboxymethylcellulose sodium, dextrose, 0.02% w/w benzalkonium chloride, polysorbate 80, and 0.25% w/w phenylethyl alcohol, and has a pH between 5 and 7.
- It is necessary to prime the pump before first use or after a period of non-use (1 week or more). After initial priming (6 actuations), each actuation delivers 50 mcg of fluticasone propionate in 100 mg of formulation through the nasal adapter. Each 16-g bottle of fluticasone nasal spray provides 120 metered sprays. After 120 metered sprays, the amount of fluticasone propionate delivered per actuation may not be consistent and the unit should be discarded.
Lotion/Spray
- Fluticasone propionate is a white to off-white powder with a molecular weight of 500.6. It is practically insoluble in water, freely soluble in dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol.
- Each gram of fluticasone contains 0.5mg fluticasone propionate in a base of cetostearyl alcohol, isopropyl myristate, propylene glycol, cetomacrogol 1000, dimethicone 350, citric acid, sodium citrate, and purified water, with imidurea, methylparaben, and propylparaben as preservatives.
Pharmacodynamics
Aerosol
- Serum cortisol concentrations, urinary excretion of cortisol, and urine 6-β-hydroxycortisol excretion collected over 24 hours in 24 healthy subjects following 8 inhalations of fluticasone propionate HFA 44, 110, and 220 mcg decreased with increasing dose. However, in patients with asthma treated with 2 inhalations of fluticasone propionate HFA 44, 110, and 220 mcg twice daily for at least 4 weeks, differences in serum cortisol AUC(0-12 h) (n = 65) and 24-hour urinary excretion of cortisol (n = 47) compared with placebo were not related to dose and generally not significant. In the study with healthy volunteers, the effect of propellant was also evaluated by comparing results following the 220-mcg strength inhaler containing HFA 134a propellant with the same strength of inhaler containing CFC 11/12 propellant. A lesser effect on the HPA axis with the HFA formulation was observed for serum cortisol, but not urine cortisol and 6-betahydroxy cortisol excretion. In addition, in a crossover study of children with asthma aged 4 to 11 years (N = 40), 24-hour urinary excretion of cortisol was not affected after a 4-week treatment period with 88 mcg of fluticasone propionate HFA twice daily compared with urinary excretion after the 2-week placebo period. The ratio (95% CI) of urinary excretion of cortisol over 24 hours following fluticasone propionate HFA versus placebo was 0.987 (0.796, 1.223).
- The potential systemic effects of fluticasone propionate HFA on the HPA axis were also studied in patients with asthma. Fluticasone propionate given by inhalation aerosol at dosages of 440 or 880 mcg twice daily was compared with placebo in oral corticosteroid-dependent patients with asthma (range of mean dose of prednisone at baseline: 13 to 14 mg/day) in a 16-week study. Consistent with maintenance treatment with oral corticosteroids, abnormal plasma cortisol responses to short cosyntropin stimulation (peak plasma cortisol less than 18 mcg/dL) were present at baseline in the majority of patients participating in this study (69% of patients later randomized to placebo and 72% to 78% of patients later randomized to fluticasone propionate HFA). At week 16, 8 patients (73%) on placebo compared with 14 (54%) and 13 (68%) patients receiving fluticasone propionate HFA (440 and 880 mcg twice daily, respectively) had post-stimulation cortisol levels of less than 18 mcg/dL.
Nasal Spray
- In a trial to evaluate the potential systemic and topical effects of fluticasone nasal spray on allergic rhinitis symptoms, the benefits of comparable drug blood levels produced by fluticasone nasal spray and oral fluticasone propionate were compared. The dosages used were 200 mcg of Fluticasone nasal spray, the nasal spray vehicle (plus oral placebo), and 5 and 10 mg of oral fluticasone propionate (plus nasal spray vehicle) per day for 14 days. Plasma levels were undetectable in the majority of patients after intranasal dosing, but present at low levels in the majority after oral dosing. Fluticasone nasal spray was significantly more effective in reducing symptoms of allergic rhinitis than either the oral fluticasone propionate or the nasal vehicle. This trial demonstrated that the therapeutic effect of fluticasone nasal spray can be attributed to the topical effects of fluticasone propionate.
- In another trial, the potential systemic effects of fluticasone nasal spray on the hypothalamic-pituitary-adrenal (HPA) axis were also studied in allergic patients. Fluticasone nasal spray given as 200 mcg once daily or 400 mcg twice daily was compared with placebo or oral prednisone 7.5 or 15 mg given in the morning. Fluticasone nasal spray at either dosage for 4 weeks did not affect the adrenal response to 6-hour cosyntropin stimulation, while both dosages of oral prednisone significantly reduced the response to cosyntropin.
Lotion/Spray
- Like other topical corticosteroids, fluticasone propionate has anti-inflammatory, antipruritic, and vasoconstrictive properties.
- Although fluticasone propionate has a weak affinity for the progesterone receptor and virtually no affinity for the mineralocorticoid, estrogen or androgen receptors, the clinical relevance as related to safety is unknown. Fluticasone propionate is lipophilic and has strong affinity for the glucocorticoid receptor. The therapeutic potency of glucocorticoids is related to the half-life of the glucocorticoid receptor complex. The half-life of the fluticasone propionate-glucocorticoid receptor complex is approximately 10 hours.
Pharmacokinetics
Aerosol
- Absorption: Fluticasone propionate acts locally in the lung; therefore, plasma levels do not predict therapeutic effect. Studies using oral dosing of labeled and unlabeled drug have demonstrated that the oral systemic bioavailability of fluticasone propionate is negligible (less than 1%), primarily due to incomplete absorption and presystemic metabolism in the gut and liver. In contrast, the majority of the fluticasone propionate delivered to the lung is systemically absorbed.
- Distribution: Following intravenous administration, the initial disposition phase for fluticasone propionate was rapid and consistent with its high lipid solubility and tissue binding. The volume of distribution averaged 4.2 L/kg.
- The percentage of fluticasone propionate bound to human plasma proteins averages 99%. Fluticasone propionate is weakly and reversibly bound to erythrocytes and is not significantly bound to human transcortin.
- Metabolism: The total clearance of fluticasone propionate is high (average, 1,093 mL/min), with renal clearance accounting for less than 0.02% of the total. The only circulating metabolite detected in man is the 17β-carboxylic acid derivative of fluticasone propionate, which is formed through the CYP 3A4 pathway. This metabolite had less affinity (approximately 1/2,000) than the parent drug for the corticosteroid receptor of human lung cytosol in vitro and negligible pharmacological activity in animal studies. Other metabolites detected in vitro using cultured human hepatoma cells have not been detected in man.
- Elimination: Following intravenous dosing, fluticasone propionate showed polyexponential kinetics and had a terminal elimination half-life of approximately 7.8 hours. Less than 5% of a radiolabeled oral dose was excreted in the urine as metabolites, with the remainder excreted in the feces as parent drug and metabolites.
=Pediatrics
- A population pharmacokinetic analysis was performed for fluticasone using steady-state data from 4 controlled clinical trials and single-dose data from 1 controlled clinical trial. The combined cohort for analysis included 269 patients (161 males and 108 females) with asthma aged 6 months to 66 years who received treatment with fluticasone. Most of these subjects (n = 215) were treated with fluticasone 44 mcg given as 88 mcg twice daily. Fluticasone was delivered using an AeroChamber Plus VHC with a mask to patients aged younger than 4 years. Data from adult patients with asthma following fluticasone 110 mcg given as 220 mcg twice daily (n = 15) and following fluticasone 220 mcg given as 440 mcg twice daily (n = 17) at steady state were also included. Data for 22 patients came from a single-dose crossover study of 264 mcg (6 doses of fluticasone 44 mcg) with and without AeroChamber Plus VHC in children with asthma aged 4 to 11 years.
- Stratification of exposure data following fluticasone 88 mcg by age and study indicated that systemic exposure to fluticasone propionate at steady state was similar in children aged 6 to younger than 12 months, children aged 1 to younger than 4 years, and adults and adolescents aged 12 years and older. Exposure was lower in children aged 4 to 11 years, who did not use a VHC, as shown in Table 4.
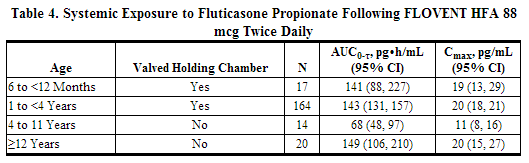
- The lower exposure to fluticasone propionate in children aged 4 to 11 years who did not use a VHC may reflect the inability to coordinate actuation and inhalation of the metered-dose inhaler. The impact of the use of a VHC on exposure to fluticasone propionate in patients aged 4 to 11 years was evaluated in a single-dose crossover study with fluticasone 44 mcg given as 264 mcg. In this study, use of a VHC increased systemic exposure to fluticasone propionate (table below), possibly correcting for the inability to coordinate actuation and inhalation.

- There was a dose-related increase in systemic exposure in patients aged 12 years and older receiving higher doses of fluticasone propionate (220 and 440 mcg twice daily). The AUC0-τ in pg•h/mL was 358 (95% CI: 272, 473) and 640 (95% CI: 477, 858), and Cmax in pg/mL was 47.3 (95% CI: 37, 61) and 87 (95% CI: 68, 112) following fluticasone propionate 220 and 440 mcg, respectively.
Nasal Spray
- Absorption: The activity of fluticasone nasal spray is due to the parent drug, fluticasone propionate. Indirect calculations indicate that fluticasone propionate delivered by the intranasal route has an absolute bioavailability averaging less than 2%. After intranasal treatment of patients with allergic rhinitis for 3 weeks, fluticasone propionate plasma concentrations were above the level of detection (50 pg/mL) only when recommended doses were exceeded and then only in occasional samples at low plasma levels. Due to the low bioavailability by the intranasal route, the majority of the pharmacokinetic data was obtained via other routes of administration. Studies using oral dosing of radiolabeled drug have demonstrated that fluticasone propionate is highly extracted from plasma and absorption is low. Oral bioavailability is negligible, and the majority of the circulating radioactivity is due to an inactive metabolite.
- Distribution: Following intravenous administration, the initial disposition phase for fluticasone propionate was rapid and consistent with its high lipid solubility and tissue binding. The volume of distribution averaged 4.2 L/kg.
- The percentage of fluticasone propionate bound to human plasma proteins averaged 91% with no obvious concentration relationship. Fluticasone propionate is weakly and reversibly bound to erythrocytes and freely equilibrates between erythrocytes and plasma. Fluticasone propionate is not significantly bound to human transcortin.
- Metabolism: The total blood clearance of fluticasone propionate is high (average, 1,093 mL/min), with renal clearance accounting for less than 0.02% of the total. The only circulating metabolite detected in man is the 17β-carboxylic acid derivative of fluticasone propionate, which is formed through the cytochrome P450 3A4 pathway. This inactive metabolite had less affinity (approximately 1/2,000) than the parent drug for the glucocorticoid receptor of human lung cytosol in vitro and negligible pharmacological activity in animal studies. Other metabolites detected in vitro using cultured human hepatoma cells have not been detected in man.
- Elimination: Following intravenous dosing, fluticasone propionate showed polyexponential kinetics and had a terminal elimination half-life of approximately 7.8 hours. Less than 5% of a radiolabeled oral dose was excreted in the urine as metabolites, with the remainder excreted in the feces as parent drug and metabolites.
Lotion/Spray
- Absorption: The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusive dressing enhances penetration. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption.
- Distribution: Following intravenous administration of 1 mg of fluticasone propionate in healthy volunteers, the initial disposition phase for fluticasone propionate was rapid and consistent with its high lipid solubility and tissue binding. The apparent volume of distribution averaged 4.2 L/kg (range, 2.3 to 16.7 L/kg). The percentage of fluticasone propionate bound to human plasma proteins averaged 91%. Fluticasone propionate is weakly and reversibly bound to erythrocytes. Fluticasone propionate is not significantly bound to human transcortin.
- Metabolism: No metabolites of fluticasone propionate were detected in an in vitro study of radiolabeled fluticasone propionate incubated in a human skin homogenate. The total blood clearance of systemically absorbed fluticasone propionate averages 1093 mL/min (range, 618 to 1702 mL/min) after a 1-mg intravenous dose, with renal clearance accounting for less than 0.02% of the total.
- Orally absorbed fluticasone propionate has demonstrated extensive first-pass metabolism with no unchanged drug detected in the plasma up to 6 hours after dosing. Fluticasone propionate is metabolized in the liver by cytochrome P450 3A4-mediated hydrolysis of the 5-fluoromethyl carbothiolate grouping. This transformation occurs in 1 metabolic step to produce the inactive 17β-carboxylic acid metabolite, the only known metabolite detected in man. This metabolite has approximately 2000 times less affinity than the parent drug for the glucocorticoid receptor of human lung cytosol in vitro and negligible pharmacological activity in animal studies. Other metabolites detected in vitro using cultured human hepatoma cells have not been detected in man.
- Excretion: Following an intravenous dose of 1 mg in healthy volunteers, fluticasone propionate showed polyexponential kinetics and had an average terminal half-life of 7.2 hours (range, 3.2 to 11.2 hours).
Pediatric
Plasma fluticasone levels were measured in patients 2 years - 6 years of age in an HPA axis suppression study. A total of 13 (62%) of 21 patients tested had measurable fluticasone at the end of 3 - 4 weeks of treatment. The mean ± SD fluticasone plasma values for patients aged under 3 years was 47.7 ± 31.7 pg/mL and 175.5 ± 243.6 pg/mL. Three patients had fluticasone levels over 300 pg/mL, with one of these having a level of 819.81 pg/mL. No data was obtained for patients < 2 years of age.
Nonclinical Toxicology
Aerosol
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Fluticasone propionate demonstrated no tumorigenic potential in mice at oral doses up to 1,000 mcg/kg (approximately 2 and 10 times the MRHD for adults and children aged 4 to 11 years, respectively, on a mg/m2 basis) for 78 weeks or in rats at inhalation doses up to 57 mcg/kg (approximately 0.3 times and approximately equivalent to the MRHD for adults and children aged 4 to 11 years, respectively, on a mg/m2 basis) for 104 weeks.
- Fluticasone propionate did not induce gene mutation in prokaryotic or eukaryotic cells in vitro. No significant clastogenic effect was seen in cultured human peripheral lymphocytes in vitro or in the in vivo mouse micronucleus test.
- No evidence of impairment of fertility was observed in reproductive studies conducted in male and female rats at subcutaneous doses up to 50 mcg/kg (approximately 0.2 times the MRHD for adults on a mg/m2 basis). Prostate weight was significantly reduced at a subcutaneous dose of 50 mcg/kg.
Animal Toxicology and/or Pharmacology
- Reproductive Toxicology: Subcutaneous studies in mice and rats at 45 and 100 mcg/kg (approximately 0.1 and 0.5 times the MRHD for adults on a mg/m2 basis, respectively) revealed fetal toxicity characteristic of potent corticosteroid compounds, including embryonic growth retardation, omphalocele, cleft palate, and retarded cranial ossification.
- In rabbits, fetal weight reduction and cleft palate were observed at a subcutaneous dose of 4 mcg/kg (approximately 0.04 times the MRHD for adults on a mg/m2 basis). However, no teratogenic effects were reported at oral doses up to 300 mcg/kg (approximately 3 times the MRHD for adults on a mg/m2 basis) of fluticasone propionate. No fluticasone propionate was detected in the plasma in this study, consistent with the established low bioavailability following oral administration.
- Fluticasone propionate crossed the placenta following subcutaneous administration to mice and rats and oral administration to rabbits.
- In animals and humans, propellant HFA-134a was found to be rapidly absorbed and rapidly eliminated, with an elimination half-life of 3 to 27 minutes in animals and 5 to 7 minutes in humans. Time to maximum plasma concentration (Tmax) and mean residence time are both extremely short, leading to a transient appearance of HFA-134a in the blood with no evidence of accumulation.
- Propellant HFA-134a is devoid of pharmacological activity except at very high doses in animals (i.e., 380 to 1,300 times the maximum human exposure based on comparisons of AUC values), primarily producing ataxia, tremors, dyspnea, or salivation. These events are similar to effects produced by the structurally related CFCs, which have been used extensively in metered-dose inhalers.
Nasal Spray
- Fluticasone propionate demonstrated no tumorigenic potential in mice at oral doses up to 1,000 mcg/kg (approximately 20 times the maximum recommended daily intranasal dose in adults and approximately 10 times the maximum recommended daily intranasal dose in children on a mcg/m2 basis) for 78 weeks or in rats at inhalation doses up to 57 mcg/kg (approximately 2 times the maximum recommended daily intranasal dose in adults and approximately equivalent to the maximum recommended daily intranasal dose in children on a mcg/m2 basis) for 104 weeks.
- Fluticasone propionate did not induce gene mutation in prokaryotic or eukaryotic cells in vitro. No significant clastogenic effect was seen in cultured human peripheral lymphocytes in vitro or in the mouse micronucleus test.
- No evidence of impairment of fertility was observed in reproductive studies conducted in male and female rats at subcutaneous doses up to 50 mcg/kg (approximately 2 times the maximum recommended daily intranasal dose in adults on a mcg/m2 basis). Prostate weight was significantly reduced at a subcutaneous dose of 50 mcg/kg.
Lotion/Spray
- In an oral (gavage) mouse carcinogenicity study, doses of 0.1, 0.3 and 1 mg/kg/day fluticasone propionate were administered to mice for 18 months. Fluticasone propionate demonstrated no tumorigenic potential at oral doses up to 1 mg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study.
- In a dermal mouse carcinogenicity study, 0.05% fluticasone propionate ointment (40 μl) was topically administered for 1, 3 or 7 days/week for 80 weeks. Fluticasone propionate demonstrated no tumorigenic potential at dermal doses up to 6.7 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study.
- In a 52 week dermal photo-carcinogenicity study conducted in hairless albino mice with concurrent exposure to low level ultraviolet radiation (40 weeks of treatment followed by 12 weeks of observation), topically treated lotion vehicle animals and 0.05% fluticasone propionate lotion animals demonstrated shorter time to benign skin tumor formation compared to untreated control animals. Lotion vehicle treated animals developed benign skin tumors in a shorter time than 0.05% fluticasone propionate lotion treated animals. Although applicability of results to clinical use of fluticasone in humans is unknown, and enhanced tumor growth in patients treated with fluticasone has not been reported, patients should exercise general precautions in minimizing UV light exposure by avoiding excessive or unnecessary exposure to either natural or artificial sunlight (including sunbathing, tanning booths, sun lamps, etc.)
- Fluticasone propionate revealed no evidence of mutagenic or clastogenic potential based on the results of five in vitro genotoxicity tests (Ames assay, E. coli fluctuation test, S. cerevisiae gene conversion test, Chinese hamster ovary cell chromosome aberration assay and human lymphocyte chromosome aberration assay) and one in vivo genotoxicity test (mouse micronucleus assay).
- No evidence of impairment of fertility or effect on mating performance was observed in a fertility and general reproductive performance study conducted in male and female rats at subcutaneous doses up to 50 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Clinical Studies
Aerosol
Adult and Adolescent Patients Aged 12 Years and Older
Three randomized, double-blind, parallel-group, placebo-controlled, US clinical trials were conducted in 980 adult and adolescent patients (aged 12 years and older) with asthma to assess the efficacy and safety of fluticasone in the treatment of asthma. Fixed dosages of 88, 220, and 440 mcg twice daily (each dose administered as 2 inhalations of the 44-, 110-, and 220-mcg strengths, respectively) and 880 mcg twice daily (administered as 4 inhalations of the 220-mcg strength) were compared with placebo to provide information about appropriate dosing to cover a range of asthma severity. Patients in these studies included those inadequately controlled with bronchodilators alone (Study 1), those already receiving inhaled corticosteroids (Study 2), and those requiring oral corticosteroid therapy (Study 3). In all 3 studies, patients (including placebo-treated patients) were allowed to use albuterol inhalation aerosol as needed for relief of acute asthma symptoms. In Studies 1 and 2, other maintenance asthma therapies were discontinued.
- Study 1 enrolled 397 patients with asthma inadequately controlled on bronchodilators alone. Fluticasone was evaluated at dosages of 88, 220, and 440 mcg twice daily for 12 weeks. Baseline FEV1 values were similar across groups (mean 67% of predicted normal). All 3 dosages of Fluticasone demonstrated a statistically significant improvement in lung function as measured by improvement in AM pre-dose FEV1 compared with placebo. This improvement was observed after the first week of treatment, and was maintained over the 12-week treatment period.
- At Endpoint (last observation), mean change from baseline in AM pre-dose percent predicted FEV1 was greater in all 3 groups treated with fluticasone (9.0% to 11.2%) compared with the placebo group (3.4%). The mean differences between the groups treated with fluticasone 88, 220, and 440 mcg and the placebo group were statistically significant, and the corresponding 95% confidence intervals were (2.2%, 9.2%), (2.8%, 9.9%), and (4.3%, 11.3%), respectively.
- The figure below displays results of pulmonary function tests (mean percent change from baseline in FEV1 prior to AM dose) for the recommended starting dosage of fluticasone (88 mcg twice daily) and placebo from Study 1. This trial used predetermined criteria for lack of efficacy (indicators of worsening asthma), resulting in withdrawal of more patients in the placebo group. Therefore, pulmonary function results at Endpoint (the last evaluable FEV1 result, including most patients’ lung function data) are also displayed.
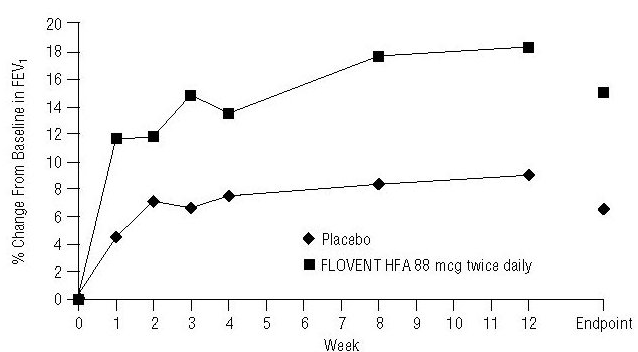
Figure: A 12-Week Clinical Trial in Patients Aged 12 Years and Older Inadequately Controlled on Bronchodilators Alone: Mean Percent Change From Baseline in FEV1 Prior to AM Dose (Study 1)
- In Study 2, fluticasone at dosages of 88, 220, and 440 mcg twice daily was evaluated over 12 weeks of treatment in 415 patients with asthma who were already receiving an inhaled corticosteroid at a daily dose within its recommended dose range in addition to as-needed albuterol. Baseline FEV1 values were similar across groups (mean 65% to 66% of predicted normal). All 3 dosages of fluticasone demonstrated a statistically significant improvement in lung function, as measured by improvement in FEV1, compared with placebo. This improvement was observed after the first week of treatment and was maintained over the 12-week treatment period. Discontinuations from the study for lack of efficacy (defined by a pre-specified decrease in FEV1 or PEF, or an increase in use of albuterol or nighttime awakenings requiring treatment with albuterol) were lower in the groups treated with fluticasone (6% to 11%) compared with placebo (50%).
- At Endpoint (last observation), mean change from baseline in AM pre-dose percent predicted FEV1 was greater in all 3 groups treated with fluticasone (2.2% to 4.6%) compared with the placebo group (-8.3%). The mean differences between the groups treated with fluticasone 88, 220, and 440 mcg and the placebo group were statistically significant, and the corresponding 95% confidence intervals were (7.1%, 13.8%), (8.2%, 14.9%), and (9.6%, 16.4%), respectively.
- Figure 2 displays the mean percent change from baseline in FEV1 from Week 1 through Week 12. This study also used predetermined criteria for lack of efficacy, resulting in withdrawal of more patients in the placebo group; therefore, pulmonary function results at Endpoint are also displayed.
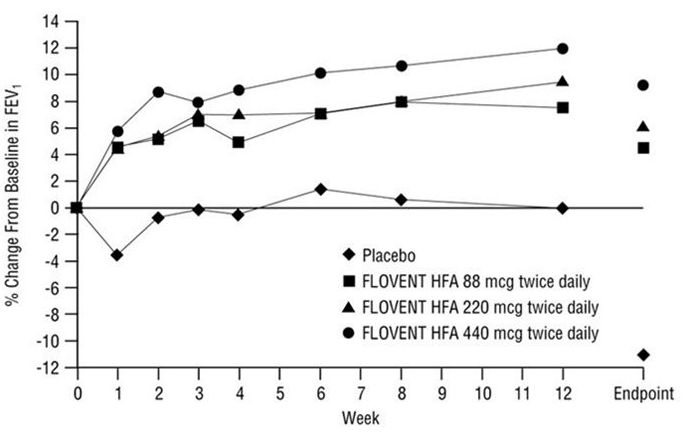
Figure: A 12-Week Clinical Trial in Patients Aged 12 Years and Older Already Receiving Daily Inhaled Corticosteroids: Mean Percent Change From Baseline in FEV1 Prior to AM Dose (Study 2)
In both studies, use of albuterol, AM and PM PEF, and asthma symptom scores showed numerical improvement with fluticasone compared with placebo.
- Study 3 enrolled 168 patients with asthma requiring oral prednisone therapy (average baseline daily prednisone dose ranged from 13 to 14 mg). Fluticasone at dosages of 440 and 880 mcg twice daily was evaluated over a 16-week treatment period. Baseline FEV1 values were similar across groups (mean 59% to 62% of predicted normal). Over the course of the study, patients treated with either dosage of fluticasone required a statistically significantly lower mean daily oral prednisone dose (6 mg) compared with placebo-treated patients (15 mg). Both dosages of fluticasone enabled a larger percentage of patients (59% and 56% in the groups treated with fluticasone 440 and 880 mcg, respectively, twice daily) to eliminate oral prednisone as compared with placebo (13%). There was no efficacy advantage of fluticasone 880 mcg twice daily compared with 440 mcg twice daily. Accompanying the reduction in oral corticosteroid use, patients treated with either dosage of fluticasone had statistically significantly improved lung function, fewer asthma symptoms, and less use of albuterol inhalation aerosol compared with the placebo-treated patients.
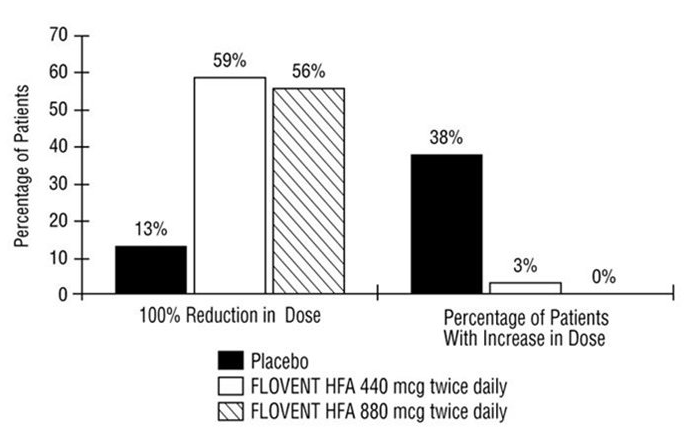
Figure: A 16-Week Clinical Trial in Patients Aged 12 Years and Older Requiring Chronic Oral Prednisone Therapy: Change in Maintenance Prednisone Dose
- Two long-term safety studies (Study 4 and Study 5) of ≥6 months’ duration were conducted in 507 adult and adolescent patients with asthma. Study 4 was designed to monitor the safety of 2 doses of fluticasone, while Study 5 compared fluticasone propionate HFA with fluticasone propionate CFC. Study 4 enrolled 182 patients who were treated daily with low to high doses of inhaled corticosteroids, beta-agonists (short-acting [as needed or regularly scheduled] or long-acting), theophylline, inhaled cromolyn or nedocromil sodium, leukotriene receptor antagonists, or 5-lipoxygenase inhibitors at baseline. Fluticasone at dosages of 220 and 440 mcg twice daily was evaluated over a 26-week treatment period in 89 and 93 patients, respectively.
- Study 5 enrolled 325 patients who were treated daily with moderate to high doses of inhaled corticosteroids, with or without concurrent use of salmeterol or albuterol, at baseline. Fluticasone propionate HFA at a dosage of 440 mcg twice daily and fluticasone propionate CFC at a dosage of 440 mcg twice daily were evaluated over a 52-week treatment period in 163 and 162 patients, respectively. Baseline FEV1 values were similar across groups (mean 81% to 84% of predicted normal). Throughout the 52-week treatment period, asthma control was maintained with both formulations of fluticasone propionate compared with baseline. In both studies, none of the patients were withdrawn due to lack of efficacy.
Pediatric Patients Aged 4 to 11 Years
A 12-week clinical trial conducted in 241 pediatric patients with asthma was supportive of efficacy but inconclusive due to measurable levels of fluticasone propionate in 6/48 (13%) of the plasma samples from patients randomized to placebo. Efficacy in patients aged 4 to 11 years is extrapolated from adult data with fluticasone and other supporting data
Nasal Spray
- A total of 13 randomized, double-blind, parallel-group, multicenter, vehicle placebo-controlled clinical trials were conducted in the United States in adults and pediatric patients (4 years of age and older) to investigate regular use of fluticasone nasal spray in patients with seasonal or perennial allergic rhinitis. The trials included 2,633 adults (1,439 men and 1,194 women) with a mean age of 37 (range, 18 to 79 years). A total of 440 adolescents (405 boys and 35 girls), mean age of 14 (range, 12 to 17 years), and 500 children (325 boys and 175 girls), mean age of 9 (range, 4 to 11 years) were also studied. The overall racial distribution was 89% white, 4% black, and 7% other. These trials evaluated the total nasal symptom scores (TNSS) that included rhinorrhea, nasal obstruction, sneezing, and nasal itching in known allergic patients who were treated for 2 to 24 weeks. Subjects treated with fluticasone nasal spray exhibited significantly greater decreases in TNSS than vehicle placebo-treated patients. Nasal mucosal basophils and eosinophils were also reduced at the end of treatment in adult studies; however, the clinical significance of this decrease is not known.
- There were no significant differences between fluticasone propionate regimens whether administered as a single daily dose of 200 mcg (two 50-mcg sprays in each nostril) or as 100 mcg (one 50-mcg spray in each nostril) twice daily in 6 clinical trials. A clear dose response could not be identified in clinical trials. In 1 trial, 200 mcg/day was slightly more effective than 50 mcg/day during the first few days of treatment; thereafter, no difference was seen.
- Two randomized, double-blind, parallel-group, multicenter, vehicle placebo-controlled 28-day trials were conducted in the United States in 732 patients (243 given fluticasone nasal spray) 12 years of age and older to investigate “as-needed” use of fluticasone nasal spray (200 mcg) in patients with seasonal allergic rhinitis. Patients were instructed to take the study medication only on days when they thought they needed the medication for symptom control, not to exceed 2 sprays per nostril on any day, and not more than once daily. “As-needed” use was prospectively defined as average use of study medication no more than 75% of study days. Average use of study medications was 57% to 70% of days for all treatment arms. The studies demonstrated significantly greater reduction in TNSS (sum of nasal congestion, rhinorrhea, sneezing, and nasal itching) with fluticasone nasal spray 200 mcg compared to placebo. The relative difference in efficacy with as-needed use as compared to regularly administered doses was not studied.
- Three randomized, double-blind, parallel-group, vehicle placebo-controlled trials were conducted in 1,191 patients to investigate regular use of fluticasone nasal spray in patients with perennial nonallergic rhinitis. These trials evaluated the patient-rated TNSS (nasal obstruction, postnasal drip, rhinorrhea) in patients treated for 28 days of double-blind therapy and in 1 of the 3 trials for 6 months of open-label treatment. Two of these trials demonstrated that patients treated with fluticasone nasal spray at a dosage of 100 mcg twice daily exhibited statistically significant decreases in TNSS compared with patients treated with vehicle.
Individualization of Dosage
- Patients should use fluticasone nasal spray at regular intervals for optimal effect.
- Adult patients may be started on a 200-mcg once-daily regimen (two 50-mcg sprays in each nostril once daily). An alternative 200-mcg/day dosage regimen can be given as 100 mcg twice daily (one 50-mcg spray in each nostril twice daily).
- Individual patients will experience a variable time to onset and different degree of symptom relief. In 4 randomized, double-blind, vehicle placebo-controlled, parallel-group allergic rhinitis studies and 2 studies of patients in an outdoor “park” setting (park studies), a decrease in nasal symptoms in treated subjects compared to placebo was shown to occur as soon as 12 hours after treatment with a 200-mcg dose of fluticasone nasal spray. Maximum effect may take several days. Regular-use patients who have responded may be able to be maintained (after 4 to 7 days) on 100 mcg/day (1 spray in each nostril once daily).
- Some patients (12 years of age and older) with seasonal allergic rhinitis may find as-needed use of fluticasone nasal spray (not to exceed 200 mcg daily) effective for symptom control. Greater symptom control may be achieved with scheduled regular use. Efficacy of as-needed use of fluticasone nasal spray has not been studied in pediatric patients under 12 years of age with seasonal allergic rhinitis, or patients with perennial allergic or nonallergic rhinitis.
- Pediatric patients (4 years of age and older) should be started with 100 mcg (1 spray in each nostril once daily). Treatment with 200 mcg (2 sprays in each nostril once daily or 1 spray in each nostril twice daily) should be reserved for pediatric patients not adequately responding to 100 mcg daily. Once adequate control is achieved, the dosage should be decreased to 100 mcg (1 spray in each nostril) daily.
- Maximum total daily doses should not exceed 2 sprays in each nostril (total dose, 200 mcg/day). There is no evidence that exceeding the recommended dose is more effective.
Lotion/Cream
Fluticasone applied once daily was superior to vehicle in the treatment of atopic dermatitis in two studies. The two studies enrolled 438 patients with atopic dermatitis aged 3 months and older, of which 169 patients were selected as having clinically significant* signs of erythema, infiltration/papulation and erosion/oozing/crusting at baseline. Table 1 presents the percentage of patients who completely cleared of erythema, infiltration/papulation and erosion/oozing/crusting at Week 4 out of those patients with clinically significant baseline signs.

How Supplied
Aerosol
- Flovent HFA 44 mcg Inhalation Aerosol is supplied in 10.6-g pressurized aluminum canisters containing 120 metered inhalations in boxes of 1 with patient instructions (NDC 0173-0718-20).
- Flovent HFA 110 mcg Inhalation Aerosol is supplied in 12-g pressurized aluminum canisters containing 120 metered inhalations in boxes of 1 with patient instructions (NDC 0173-0719-20).
- Flovent HFA 220 mcg Inhalation Aerosol is supplied in 12-g pressurized aluminum canisters containing 120 metered inhalations in boxes of 1 with patient instructions (NDC 0173-0720-20).
- Each canister is fitted with a counter and a dark orange oral actuator with a peach strapcap. The dark orange actuator supplied with FLOVENT HFA should not be used with any other product canisters, and actuators from other products should not be used with a FLOVENT HFA canister.
- The correct amount of medication in each inhalation cannot be assured after the counter reads 000, even though the canister is not completely empty and will continue to operate. The inhaler should be discarded when the counter reads 000.
- Keep out of reach of children. Avoid spraying in eyes.
- Contents Under Pressure: Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 120°F may cause bursting. Never throw into fire or incinerator.
Nasal Spray
- Fluticasone nasal Spray, 50 mcg is supplied in an amber glass bottle fitted with a white metering atomizing pump, white nasal adapter, and green dust cover in a box of 1 (NDC 0173-0453-01) with patient’s instructions for use.
- Each bottle contains a net fill weight of 16 g and will provide 120 actuations.
- Each actuation delivers 50 mcg of fluticasone propionate in 100 mg of formulation through the nasal adapter.
- The correct amount of medication in each spray cannot be assured after 120 sprays even though the bottle is not completely empty.
- The bottle should be discarded when the labeled number of actuations has been used.
Lotion/Cream
Fluticasone is supplied in:
- 60 mL bottle NDC 10337-434-60
- 120 mL bottle NDC 10337-434-04
Storage
Aerosol
- Store at 25°C (77°F); excursions permitted from 15° to 30°C (59° to 86°F).
- Store the inhaler with the mouthpiece down.
- For best results, the inhaler should be at room temperature before use.
Nasal Spray
- Store between 4° and 30°C (39° and 86°F).
Lotion/Cream
- Store between 15° and 30°C (59° and 86°F). Do not refrigerate.
- Keep container tightly sealed.
Images
Drug Images
{{#ask: Page Name::Fluticasone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Fluticasone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Aerosol
Oral Candidiasis
Patients should be advised that localized infections with Candida albicans have occurred in the mouth and pharynx in some patients. If oropharyngeal candidiasis develops, it should be treated with appropriate local or systemic (i.e., oral antifungal) therapy while still continuing therapy with fluticasone, but at times therapy with fluticasone may need to be temporarily interrupted under close medical supervision. Rinsing the mouth after inhalation is advised.
Status Asthmaticus and Acute Asthma Symptoms
Patients should be advised that fluticasone is not a bronchodilator and is not intended for use as rescue medication for acute asthma exacerbations. Acute asthma symptoms should be treated with an inhaled, short-acting beta2-agonist such as albuterol. Patients should be instructed to contact their physicians immediately if there is deterioration of their asthma.
Immunosuppression
Patients who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chickenpox or measles and if they are exposed to consult their physicians without delay. Patients should be informed of potential worsening of existing tuberculosis, fungal, bacterial, viral, or parasitic infections, or ocular herpes simplex.
Hypercorticism and Adrenal Suppression
Patients should be advised that fluticasone may cause systemic corticosteroid effects of hypercorticism and adrenal suppression. Additionally, patients should be instructed that deaths due to adrenal insufficiency have occurred during and after transfer from systemic corticosteroids. Patients should taper slowly from systemic corticosteroids if transferring to fluticasone.
Hypersensitivity Reactions, Including Anaphylaxis
Patients should be advised that hypersensitivity reactions including anaphylaxis, angioedema, urticaria, and bronchospasm may occur after administration of fluticasone. Patients should discontinue fluticasone if such reactions occur.
Reduction in Bone Mineral Density
Patients who are at an increased risk for decreased BMD should be advised that the use of corticosteroids may pose an additional risk.
Reduced Growth Velocity
Patients should be informed that orally inhaled corticosteroids, including fluticasone, may cause a reduction in growth velocity when administered to pediatric patients. Physicians should closely follow the growth of children and adolescents taking corticosteroids by any route.
Ocular Effects
Long-term use of inhaled corticosteroids may increase the risk of some eye problems (cataracts or glaucoma); regular eye examinations should be considered.
Use Daily for Best Effect
Patients should use fluticasone at regular intervals as directed. Individual patients will experience a variable time to onset and degree of symptom relief and the full benefit may not be achieved until treatment has been administered for 1 to 2 weeks or longer. Patients should not increase the prescribed dosage but should contact their physicians if symptoms do not improve or if the condition worsens. Patients should be instructed not to stop use of fluticasone abruptly. Patients should contact their physicians immediately if they discontinue use of fluticasone.
Nasal Spray
- Patients being treated with fluticasone nasal spray should receive the following information and instructions. This information is intended to aid them in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
- Patients should be warned to avoid exposure to chickenpox or measles and, if exposed, to consult their physician without delay.
- Patients should use fluticasone nasal spray at regular intervals for optimal effect. Some patients (12 years of age and older) with seasonal allergic rhinitis may find as-needed use of 200 mcg once daily effective for symptom control.
- A decrease in nasal symptoms may occur as soon as 12 hours after starting therapy with fluticasone nasal spray. Results in several clinical trials indicate statistically significant improvement within the first day or two of treatment; however, the full benefit of fluticasone nasal spray may not be achieved until treatment has been administered for several days. The patient should not increase the prescribed dosage but should contact the physician if symptoms do not improve or if the condition worsens.
- For the proper use of fluticasone nasal spray and to attain maximum improvement, the patient should read and follow carefully the patient’s instructions accompanying the product.
Lotion/Cream
Patients using fluticasone should receive the following information and instructions:
- Fluticasone is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
- Fluticasone should not be used for any disorder other than that for which it was prescribed.
- The treated skin area should not be bandaged or otherwise covered or wrapped so as to be occlusive unless directed by the physician.
- Patients should report to their physician any signs of local adverse reactions as well as non-healing or worsening of skin condition.
- Parents of pediatric patients should be advised not to use this medication in the treatment of diaper dermatitis unless directed by the physician. Fluticasone should not be applied in the diaper areas as diapers or plastic pants may constitute occlusive dressing.
- Fluticasone should not be used on the face, underarms, or groin areas unless directed by a physician.
- Fluticasone therapy should be discontinued if control is achieved before 4 weeks. If no improvement is seen within 2 weeks, contact a physician. The safety of the use of fluticasone for longer than 4 weeks has not been established.
- Patients should report to their physician if they are allergic to formaldehyde.
- Patients that apply fluticasone to exposed portions of the body should follow physician advice and routine precautions to avoid excessive or unnecessary exposure to either natural or artificial sunlight (such as sunbathing, tanning booths, sun lamps, etc.).
Precautions with Alcohol
Alcohol-Fluticasone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Cutivate
- Flonase
- Flovent
- Flovent Rotadisk
- Flovent HFA
- Flovent Diskus
Look-Alike Drug Names
- Flonase - Flovent
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Jankowski R, Klossek JM, Attali V, Coste A, Serrano E (2009). "Long-term study of fluticasone propionate aqueous nasal spray in acute and maintenance therapy of nasal polyposis". Allergy. 64 (6): 944–50. doi:10.1111/j.1398-9995.2009.01938.x. PMID 19298572.
{{#subobject:
|Label Page=Fluticasone |Label Name=FluticasonePackage1.png
}}
{{#subobject:
|Label Page=Fluticasone |Label Name=FluticasonePackage2.png
}}
{{#subobject:
|Label Page=Fluticasone |Label Name=FluticasonePackage3.png
}}
{{#subobject:
|Label Page=Fluticasone |Label Name=FluticasonePackage4.png
}}
{{#subobject:
|Label Page=Fluticasone |Label Name=FluticasonePackage5.png
}}
{{#subobject:
|Label Page=Fluticasone |Label Name=FluticasonePackage6.png
}}