Fasudil
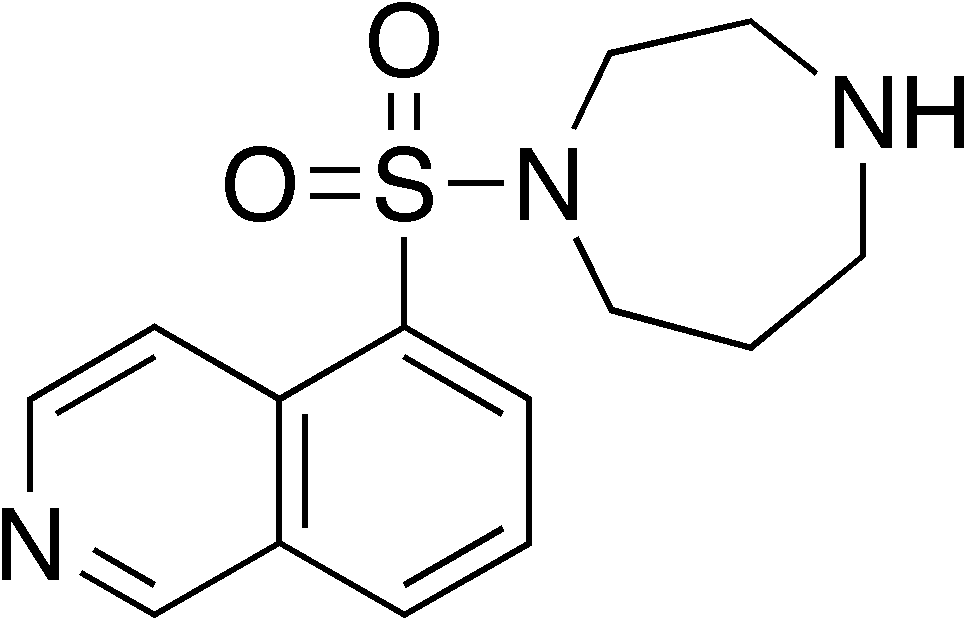 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | well absorbed |
| Metabolism | metabolized quickly to hydroxyfasudil |
| Elimination half-life | 0.76 hours. Active metabolite (hydroxyfasudil) 4.66 hours. |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C14H17N3O2S |
| Molar mass | 291.36 g/mol |
| 3D model (JSmol) | |
| |
| (verify) | |
|
WikiDoc Resources for Fasudil |
|
Articles |
|---|
|
Most recent articles on Fasudil |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Fasudil at Clinical Trials.gov Clinical Trials on Fasudil at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Fasudil
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Fasudil Risk calculators and risk factors for Fasudil
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Fasudil |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Fasudil hydrochloride (INN) is a potent Rho-kinase inhibitor and vasodilator.[1] Since it was discovered, it has been used for the treatment of cerebral vasospasm, which is often due to subarachnoid hemorrhage,[2] as well as to improve the cognitive decline seen in stroke victims. It has been found to be effective for the treatment of pulmonary hypertension.[3] It was demonstrated in February 2009 that fasudil could also be used to enhance memory and improve the prognosis of Alzheimers patients.[4]
Pulmonary Hypertension
Pulmonary hypertension (PH) is a cardiovascular disease characterized by a resting mean pulmonary arterial pressure exceeding 25 mmHg, or more than 30 mmHg during exercise.[5] It arises from the pulmonary vascular remodeling accompanied by endothelial dysfunction, fibroblast activation, and endothelial cellular proliferation. Symptoms of PH include shortness of breath, chronic fatigue, dizziness, peripheral edema, cyanosis, and chest pain. If untreated, PH could lead to heart failure and death.[6] PH is divided into 5 classifications: arterial, venous, hypoxemia, thromboembolic, and miscellaneous.[7]
Molecular Mechanism
Fasudil (HA-1077) is a selective RhoA/Rho kinase(ROCK) inhibitor.[8] ROCK is an enzyme that plays an important role in mediating vasoconstriction and vascular remodeling in the pathogenesis of PH. ROCK induces vasoconstriction by phosphorylating the myosin-binding subunit of myosin light chain (MLC) phosphatase, thus decreasing MLC phosphatase activity and enhancing vascular smooth muscle contraction.[8]
Angiotensin-converting Enzyme (ACE) expression and Angiotensin-II (Ang-II) levels
ACE is an enzyme that catalyzes the conversion of Angiotensin-I (Ang-I) to Angiotensin-II (Ang-II). Ang-II is a peptide hormone which increases blood pressure by initiating vasoconstriction and aldosterone secretion. ROCK increases ACE expression and activity in PH. By inhibiting ROCK with fasudil, circulating ACE and Ang-II are reduced, leading to a decrease in pulmonary vascular pressure.[9]
Endothelial Nitric Oxide Synthase (eNOS) expression
eNOS mediates the production of the vasodilator Nitric oxide (NO). Pulmonary arterial cell cultures treated with fasudil showed a significant increase in eNOS mRNA levels in a dose dependent manner, and the half-life of eNOS mRNA increased 2-folds. These findings suggested that ROCK inhibition with fasudil increases eNOS expression by stabilizing eNOS mRNA, which contributed to an increase of NO level to enhance vasodilation.[10]
Extracellular signal-regulated kinase(ERK) activity and p27Kip1 levels
The proliferative effects of ROCK on vascular endothelial cells is due to the activation of extracellular signal-regulated kinase (ERK).[11] ERK mediates cell proliferation via the phosphorylation of p27Kip1, thus accelerating the degradation rate of p27Kip1.[12] p27Kip1 is a cyclin-dependent kinase (CDK) inhibitor which down-regulates cell cycle by binding cyclin-CDK complex.[13] Human pulmonary arterial smooth muscle cells treated with fasudil showed a decrease in cell proliferation in a dose-dependent manner. Fasudil also decreases ERK activities, as well as increases level of p27Kip1. This suggested that the anti-proliferative effects of fasudil is due to the decrease of ERK activities via the inhibition of ROCK.[11]
References
- ↑ "Drug Found That Could Reduce Risk Of Alzheimer's". Science Daily.
- ↑ Shibuya M, Suzuki Y (September 1993). "[Treatment of cerebral vasospasm by a protein kinase inhibitor AT 877]". No to Shinkei (in Japanese). 45 (9): 819–24. PMID 8217408.
- ↑ Doggrell SA (2005). "Rho-kinase inhibitors show promise in pulmonary hypertension". Expert Opin Investig Drugs. 14 (9): 1157–1159. doi:10.1517/13543784.14.9.1157. PMID 16144499.
- ↑ Huentelman, Matthew J.; Stephan, DA; Talboom, J; Corneveaux, JJ; Reiman, DM; Gerber, JD; Barnes, CA; Alexander, GE; Reiman, EM (2009). "Peripheral delivery of a ROCK inhibitor improves learning and working memory". Behavioral Neuroscience. 123 (1): 218. doi:10.1037/a0014260. PMC 2701389. PMID 19170447.
- ↑ Badesch, D.B., Champion, H.C., Sanchez, M.A.G., Hoeper, M.M., Hoyd, J.E., Manes, A., McGoon, M., Naeije, R., Olschewski, H., Oudiz, R.J., and Torbicki, A. (2009). Diagnosis and Assessment of Pulmonary Arterial Hypertension. Journal of the American College of Cardiology. 54:55-66.
- ↑ Zeller, J.L., Burke, A.E., and Glass, R.M. (2008). Pulmonary Hypertension. The Journal of the American Medical Association. 299:3.
- ↑ Simonneau, G., Galie, M., Rubin, L.J., Langleben, D., Seeger, W., Domenighetti, G., Gibbs, S., Lebrec, D., Speich, R., Beghetti, M., Rich, S., and Fishman, A. (2004). Clinical Classification of Pulmonary Hypertension. Journal of the American College of Cardiology. 43:5-12.
- ↑ 8.0 8.1 Nagumo, H., Sasaki, Y., Ono, Y., Okamato, H., Seto, M., and Takuwa, Y. (2000). Rho kinase inhibitor HA-1077 prevents Rho-mediated myosin phosphatase inhibition in smooth muscle cells. American Journal of Physiology. 278: C57-C65.
- ↑ Ocaranze M.P., Prvera, P., Novoa, U., Pinto, M., Gonzalez, L., Chiong, M., Lavandero, S., and Jalil, J.E. (2011). Rho kinase inhibition activates the homologous angiotensin-converting enzyme-angiotensin-(1-9) axis in experimental hypertension. Journal of Hypertension. 29:706-715.
- ↑ Takemoto, M., Sun, J., Hiroko, J., Shimokawa, H., and Liao, J.K. (2002). Rho-Kinase Mediates Hypoxia-Induced Downregulation of Endothelial Nitric Oxide Synthase. Journal of the American Heart Association. 106:57-62.
- ↑ 11.0 11.1 Liu, A., Ling, F., Wang, D., Wang, Q., Lu, X., and Liu, Y. (2011). Fasudil inhibits platelet-derived growth factor-induced human pulmonary artery smooth muscle cell proliferation by up-regulation of p27kip1 via the ERK signal pathway. Chinese Medical Journal. 124:3098-3104.
- ↑ Delmas, C., Manenti, S., Boudjelal, A., Peyssonnaux, C., Eychene, A., and Darbon, J.R. (2001). The p42/p44 Mitoen-activated protein kinase activation triggers p27kip1 degradation independently of CDK2/Cyclin E in NIH 3T3 cells.The Journal of Biological Chemistry. 276:34958-34965.
- ↑ Rodman D.M., and Fouty, B.W. (2003). Mevastatin can cause G1 arrest and induce apoptosis in pulmonary artery smooth muscle cells through a p27kip1-independent pathway. Journal of the American Heart Associations. 92:501-509.
- Pages with script errors
- CS1 maint: Unrecognized language
- Template:drugs.com link with non-standard subpage
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without InChI source
- Drugs with no legal status
- Vasodilators
- Cardiovascular Drugs
- Drug
- Sulfonamides