Ethylmorphine
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C19H23NO3 |
| Molar mass | 313.391 |
|
WikiDoc Resources for Ethylmorphine |
|
Articles |
|---|
|
Most recent articles on Ethylmorphine Most cited articles on Ethylmorphine |
|
Media |
|
Powerpoint slides on Ethylmorphine |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Ethylmorphine at Clinical Trials.gov Trial results on Ethylmorphine Clinical Trials on Ethylmorphine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Ethylmorphine NICE Guidance on Ethylmorphine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Ethylmorphine Discussion groups on Ethylmorphine Patient Handouts on Ethylmorphine Directions to Hospitals Treating Ethylmorphine Risk calculators and risk factors for Ethylmorphine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Ethylmorphine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
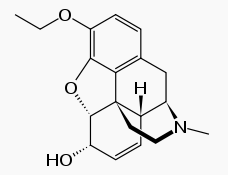
Ethylmorphine (also known as codethyline, Dionine, and ethyl morphine) is a drug in the class of both opiates (representing a minor synthetic change from morphine) and opioids (being effective in the CNS's opioid reception system). Its effects in humans mainly stem from its metabolic conversion to morphine. Chemically, it is a morphine molecule with a -Template:OxygenTemplate:Carbon2Template:Hydrogen5 group substituted for the aromatic 3-Template:Hydroxyl group. Therefore the closest chemical relative of ethylmorphine is codeine, also known as methylmorphine. Ethylmorphine also has a hydromorphone analogue (ethyldihydromorphinone or 3-ethoxy-7,8-dihydro-morphin-6-one), and a dihydromorphine analogue known as ethyldihydromorphine, although none of them appears to be commercially distributed at the current time. In most countries and internationally ethylmorphine and codeine are regulated much the same way.
Metabolism
After ingestion, ethylmorphine is converted to morphine in the human liver by the CYP450-isozyme CYP2D6, similarly to codeine. Morphine is a powerful analgesic and the main active compound found in naturally occurring opium. Ethylmorphine's metabolism is hindered by ethanol [2], which, being a CNS depressant, also boosts the drug's sedative effect on the central nervous system - creating a potentially dangerous combination as both drugs cause depression of the respiratory system that can be mutually amplified.
Medical uses
Ethylmorphine is used as an antitussive to treat dry cough. It is also a moderately strong analgesic.
Dosage
Ethylmorphine is 'less potent than morphine' but more potent than codeine' by a small percentage. Medical oral dosages vary from 5 to 30, even 50 mg. Like codeine and close chemical relatives, ethylmorphine should not be injected directly into the veins as the sudden, huge, histamine release can have dangerous impacts, Naturally, all doses are much lower in parenteral use. Ethylmorphine shares codeine's good oral bioavailability and is the fifth- or sixth-most-commonly used drug of the codeine-based semi-synthetic group of moderate to strong narcotic analgesics. This series includes codeine, dihydrocodeine, ethylmorphine, dihydroethylmorphine, benzylmorphine (Peronine®), acetyldihydrocodeine, nicocodeine, nicodicodeine, hydrocodone, thebacon, oxycodone, acetylcodone, methyldihydromorphine and others.
The lethal dose is unknown. One source (in Finnish), however, suggests it to be as low as 500 mg.
Problems
Tolerance to the drug's effects develops fast. That is why ethylmorphine is normally used only as a temporary medicine to treat e.g. cough. Patients may develop addiction. Side effects, which are rare for medical doses but normal for recreational doses, include the classical opiate side-effects: nausea, vomiting, urinary retention, miosis and constipation. Also, some people are hypersensitive or allergic to ethylmorphine and should never take it. Additionally, the same dose of ethylmorphine can have completely different effects on two different people because of large individual differences in metabolism.
Opioids are known of causing severe physical addiction, in addition to psychological addiction. This type of addiction is hard to treat.
Taking ethylmorphine in combination with alcohol or other drugs that have a suppressive effect on the central nervous system boosts both drugs' effects, creating a dangerous combination. Possible outcome is death through respiratory arrest.
In recreational use the most common problem, however, is liver damage and other effects caused by other compounds besides ethylmorphine. Some analgesics with ethylmorphine also contain indometacin (e.g. Indalgin), which is toxic in high doses.
Antidepressants such as fluoxetine (Prozac) inhibit the enzyme that metabolizes ethylmorphine. Taking ethylmorphine while using such an antidepressant may therefore lead to major changes in ethylmorphine's effects. Conversely, barbiturate compounds such as phenobarbitone induce the same enzyme, which rapidly increases the metabolism of ethylmorpine. Other current medications therefore always have to be taken into account when using this compound.
Brand names
Analgesics
- Indalgin (with indometacin)
Antitussives
- Cocillana
- Feco Syrup
See also
External links
Template:Opioids Template:Cough and cold preparations hu:Etilmorfin sv:Etylmorfin
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Drug
- Opioids
- Drugs