Ergoloid
 | |
| Combination of | |
|---|---|
| Dihydroergocristine | Ergot alkaloid |
| Dihydroergocornine | Ergot alkaloid |
| alpha-Dihydroergocryptine | Ergot alkaloid |
| beta-Dihydroergocryptine | Ergot alkaloid |
| [[{{{component5}}}]] | ? Class |
| Clinical data | |
| Synonyms | Co-dergocrine, dihydroergotoxine |
| Pregnancy category |
|
| Routes of administration | Oral, parenteral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 25% |
| Protein binding | 98–99% |
| Metabolism | 50% |
| Elimination half-life | 3.5 hours |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider | |
| ChEBI | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| | |
|
WikiDoc Resources for Ergoloid |
|
Articles |
|---|
|
Most recent articles on Ergoloid |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Ergoloid at Clinical Trials.gov Clinical Trials on Ergoloid at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Ergoloid
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Ergoloid Risk calculators and risk factors for Ergoloid
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Ergoloid |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
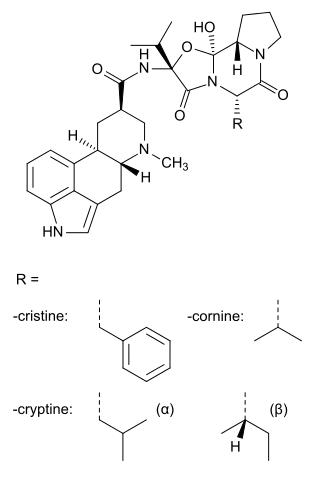
Ergoloid mesylates (USAN), co-dergocrine mesilate (BAN) or dihydroergotoxine mesylate, trade name Hydergine, is a mixture of the methanesulfonate salts of three dihydrogenated ergot alkaloids (dihydroergocristine, dihydroergocornine, and alpha- and beta-dihydroergocryptine).
It was developed by Albert Hofmann (the inventor of LSD) for Sandoz (now part of Novartis).
Uses
It has been used to treat dementia and age-related cognitive impairment (such as in Alzheimer disease),[1] as well as to aid in recovery after stroke.
There is some evidence suggesting that potentially effective doses may be higher than those currently approved in dementia treatment.[2]
Ergoloids are also used by many people as a nootropic. It may be used in conjunction with other cerebral enhancers like piracetam, with which it may act synergistically.[3]
Ergoloid Mesylate Tablets USP for sublingual use contain 1 mg of Ergoloid Mesylates USP, a mixture of the methanesulfonate salt of the following hydrogenated alkaloids: Dihydroergocornine mesylate 0.333 mg, Dihydroergocristine mesylate 0.333 mg, Dihydroergocryptine mesylate 0.333 mg.[4]
Mechanism of action
Despite the fact that hydergine has been used in the treatment of dementia for many years, its mechanism of action is still not clear.[5] It stimulates dopaminergic and serotonergic receptors and blocks alpha-adrenoreceptors.[6] Current studies imply that the major effect of hydergine may be the modulation of synaptic neurotransmission rather than solely increasing blood flow as was once thought.[7] A prominent feature that accompanies aging is an increase in monoamine oxidase (MAO) levels.[8] This results in decreased availability of catecholamines in the synaptic cleft. In one study, an interaction between age and hydergine treatment was observed in the hypothalamus, hippocampus and cerebellum. The hydergine effect was more pronounced in the aged group in the hypothalamus and cerebellum, and more pronounced in the adult in the hippocampus. These findings imply that increased brain MAO activity in aging can be modified by hydergine treatment in some brain regions.
Contraindications
Ergoloid is contraindicated in individuals who have previously shown hypersensitivity to the drug. They are also contraindicated in patients who have psychosis, acute or chronic, regardless of etiology.[9] Specific drug interactions are unknown but it has been claimed that there are multiple potential interactions.[9]
Adverse reactions
Adverse effects are minimal. The most common include transient, dose dependent nausea and gastrointestinal disturbances,[5] and sublingual irritation with SL tablets. Other common side effects include:[9][10]
- Cardiovascular: orthostatic hypotension, bradycardia
- Dermatologic: skin rash, flushing
- Ocular: blurred vision
- Respiratory: nasal congestion
- Increased risk of fibrosis and ergotism.[11][12]
As a result of the last-mentioned effects, the use of ergot derivatives for the treatment of blood circulation disorders, memory problems, sensation problems and the treatment of migraine is no longer permitted in some EU countries because the risks are believed to outweigh any benefits.[11]
Chemistry
The four constituents differ only in which of four proteinogenic amino acids is used in biosynthesis:[13]
| Compound | Amino acid |
|---|---|
| Dihydroergocristine | Phenylalanine |
| Dihydroergocornine | Valine |
| alpha-Dihydroergocryptine | Leucine |
| beta-Dihydroergocryptine | Isoleucine |
Trade names
Hydergine, Hydergina, Gerimal, Niloric, Redizork, Alkergot, Cicanol, Redergin.
References
- ↑ Flynn, B. L.; Ranno, A. E. (February 1999). "Pharmacologic Management of Alzheimer Disease, Part II: Antioxidants, Antihypertensives, and Ergoloid Derivatives". Annals of Pharmacotherapy. 33 (2): 188–197. doi:10.1345/aph.17172. PMID 10084415.
- ↑ Schneider, L. S.; Olin, J. T. (August 1994). "Overview of Clinical Trials of Hydergine in Dementia". Archives of Neurology. 51 (8): 787–798. doi:10.1001/archneur.1994.00540200063018. PMID 8042927.
- ↑ Pearson, D.; Shaw, S. (1982). "Part III Chapter 2 : Revitalizing Your Brain Power". Life Extension - A Practical Scientific Approach. Warner Books. ISBN 0-446-51229-X.
- ↑ Ergoloid (Drugs.com http://www.drugs.com/pro/ergoloid.html). Retrieved 08-02-2013.
- ↑ 5.0 5.1 Schiff, Paul L, Jr (2006) "Ergot and Its Alkaloids", American Journal of Pharmaceutical Education 70.5 p.98.
- ↑ Markstein, R. (1985). "Hydergine: Interaction with the Neurotransmitter Systems in the Central Nervous System". Journal of Pharmacology. 16 (Suppl 3): 1–17. PMID 2869188.
- ↑ Rowell et al. (Jul 1999) "Ergocryptine and other ergot alkaloids stimulate the release of [3H] dopamine from rat striatal synaptosomes", Journal of Animal Science 77.7 pp.1800-6
- ↑ Kennedy et al. (Dec 2000) "Suicide and Aging: International Perspectives", Psychiatric Quarterly 71.4 pp.345-62
- ↑ 9.0 9.1 9.2 "Drugs to Treat Alzheimer's Disease" (Apr 2014) Journal of Psychosocial Nursing & Mental Health Services 52.4 pp. 21-2
- ↑ Majumdar et al (Jul-Sep 2013) "Hyperprolactinemia", Journal of Human Reproductive Sciences 6.3 pp.168-175
- ↑ 11.0 11.1 "Ergot derivatives: restricted use" (2013) WHO Drug Information 27.3 p.225
- ↑ Helsen et al. (2013) "Ergotamine-Induced Pleural and Pericardial Effusion Successfully Treated with Colchicine", Acta Clinica Belgica 68.2 pp.113-5
- ↑ Steinhilber, D.; Schubert-Zsilavecz, M.; Roth, H. J. (2005). Medizinische Chemie (in German). Stuttgart, Germany: Deutscher Apotheker Verlag. p. 142. ISBN 3-7692-3483-9.
External links
Template:Anti-dementia drugs Template:Peripheral vasodilators Template:Ergolines
- Pages with script errors
- CS1 maint: Multiple names: authors list
- CS1 maint: Unrecognized language
- Drugs with non-standard legal status
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugboxes which contain changes to verified fields
- Infobox drug tracked parameters
- Drugs that are a combination of chemicals
- Antidementia agents
- Vasodilators
- Cardiovascular Drugs
- Drug