Empagliflozin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Empagliflozin is a sodium-glucose co-transporter 2 (SGLT2) inhibitor that is FDA approved for the treatment of adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Common adverse reactions include urinary tract infections, female genital mycotic infections, hypoglycemia, hypotension.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indicatios
- JARDIANCE is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Limitation of Use
- JARDIANCE is not recommended for patients with type 1 diabetes or for the treatment of diabetic ketoacidosis.
Dosage
- The recommended dose of JARDIANCE is 10 mg once daily in the morning, taken with or without food. In patients tolerating JARDIANCE, the dose may be increased to 25 mg .
- In patients with volume depletion, correcting this condition prior to initiation of JARDIANCE is recommended.
Patients with Renal Impairment
- Assessment of renal function is recommended prior to initiation of JARDIANCE and periodically thereafter.
- JARDIANCE should not be initiated in patients with an eGFR less than 45 mL/min/1.73 m2.
- No dose adjustment is needed in patients with an eGFR greater than or equal to 45 mL/min/1.73 m2.
- JARDIANCE should be discontinued if eGFR is persistently less than 45 mL/min/1.73 m2
DOSAGE FORMS AND STRENGTHS
- JARDIANCE (empagliflozin) 10 mg tablets are pale yellow, round, biconvex and bevel-edged, film-coated tablets debossed with “S 10” on one side and the Boehringer Ingelheim company symbol on the other side.
- JARDIANCE (empagliflozin) 25 mg tablets are pale yellow, oval, biconvex, film-coated tablets debossed with “S 25” on one side and the Boehringer Ingelheim company symbol on the other side.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Empagliflozin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Empagliflozin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Empagliflozin in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Empagliflozin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Empagliflozin in pediatric patients.
Contraindications
- History of serious hypersensitivity to JARDIANCE.
- Severe renal impairment, end-stage renal disease, or dialysis
Warnings
Hypotension
- JARDIANCE causes intravascular volume contraction. Symptomatic hypotension may occur after initiating JARDIANCE particularly in patients with renal impairment, the elderly, in patients with low systolic blood pressure, and in patients on diuretics. Before initiating JARDIANCE, assess for volume contraction and correct volume status if indicated. Monitor for signs and symptoms of hypotension after initiating therapy and increase monitoring in clinical situations where volume contraction is expected .
Impairment in Renal Function
- JARDIANCE increases serum creatinine and decreases eGFR . The risk of impaired renal function with JARDIANCE is increased in elderly patients and patients with moderate renal impairment. More frequent monitoring of renal function is recommended in these patients. Renal function should be evaluated prior to initiating JARDIANCE and periodically thereafter.
Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
- Insulin and insulin secretagogues are known to cause hypoglycemia. The risk of hypoglycemia is increased when JARDIANCE is used in combination with insulin secretagogues (e.g., sulfonylurea) or insulin. Therefore, a lower dose of the insulin secretagogue or insulin may be required to reduce the risk of hypoglycemia when used in combination with JARDIANCE.
Genital Mycotic Infections
- JARDIANCE increases the risk for genital mycotic infections. Patients with a history of chronic or recurrent genital mycotic infections were more likely to develop mycotic genital infections. Monitor and treat as appropriate.
Urinary Tract Infections
- JARDIANCE increases the risk for urinary tract infections. Monitor and treat as appropriate.
Increased Low-Density Lipoprotein Cholesterol (LDL-C)
- Increases in LDL-C can occur with JARDIANCE. Monitor and treat as appropriate.
Macrovascular Outcomes
- There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with JARDIANCE or any other antidiabetic drug.
Adverse Reactions
Clinical Trials Experience
- The following important adverse reactions are described below and elsewhere in the labeling:
- Hypotension
- Impairment in Renal Function
- Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
- Genital Mycotic Infections
- Urinary Tract Infections
- Increased Low-Density Lipoprotein Cholesterol (LDL-C)
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
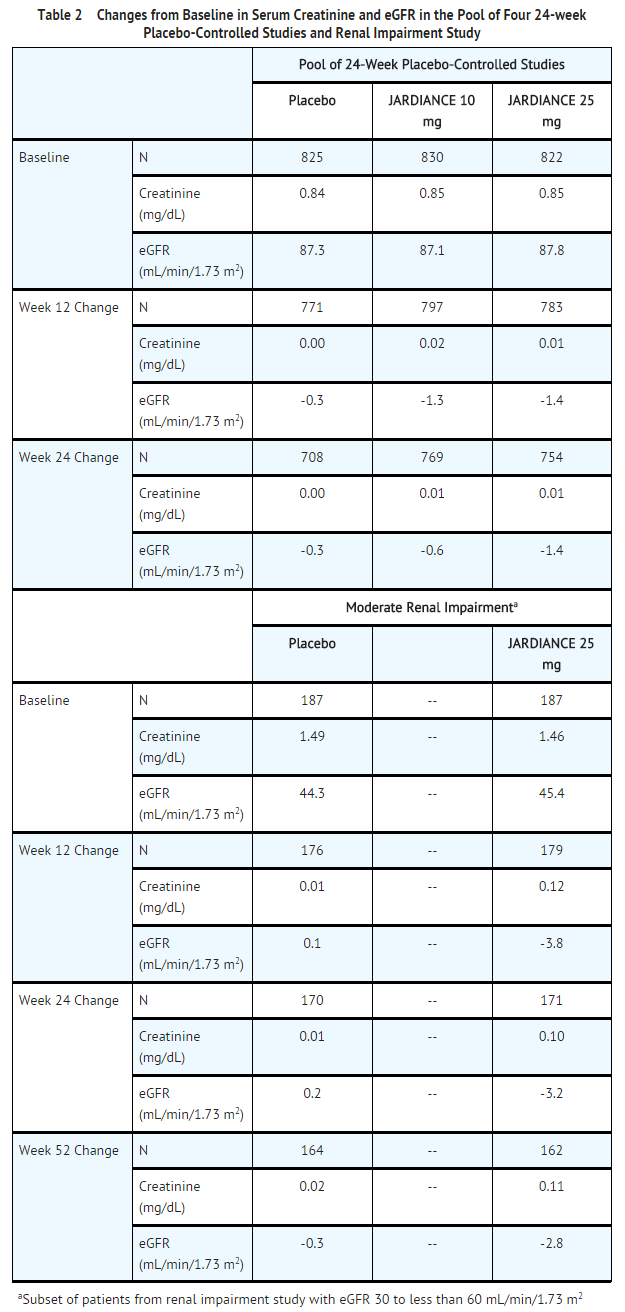
Pool of Placebo-Controlled Trials evaluating JARDIANCE 10 and 25 mg
- The data in Table 1 are derived from a pool of four 24-week placebo-controlled trials and 18-week data from a placebo-controlled trial with insulin. JARDIANCE was used as monotherapy in one trial and as add-on therapy in four trials .
- These data reflect exposure of 1976 patients to JARDIANCE with a mean exposure duration of approximately 23 weeks. Patients received placebo (N=995), JARDIANCE 10 mg (N=999), or JARDIANCE 25 mg (N=977) once daily. The mean age of the population was 56 years and 3% were older than 75 years of age. More than half (55%) of the population was male; 46% were White, 50% were Asian, and 3% were Black or African American. At baseline, 57% of the population had diabetes more than 5 years and had a mean hemoglobin A1c (HbA1c) of 8%. Established microvascular complications of diabetes at baseline included diabetic nephropathy (7%), retinopathy (8%), or neuropathy (16%). Baseline renal function was normal or mildly impaired in 91% of patients and moderately impaired in 9% of patients (mean eGFR 86.8 mL/min/1.73 m2).
- Table 1 shows common adverse reactions (excluding hypoglycemia) associated with the use of JARDIANCE. The adverse reactions were not present at baseline, occurred more commonly on JARDIANCE than on placebo and occurred in greater than or equal to 2% of patients treated with JARDIANCE 10 mg or JARDIANCE 25 mg.
- Thirst (including polydipsia) was reported in 0%, 1.7%, and 1.5% for placebo, JARDIANCE 10 mg, and JARDIANCE 25 mg, respectively.
Volume Depletion
- JARDIANCE causes an osmotic diuresis, which may lead to intravascular volume contraction and adverse reactions related to volume depletion. In the pool of five placebo-controlled clinical trials, adverse reactions related to volume depletion (e.g., blood pressure (ambulatory) decreased, blood pressure systolic decreased, dehydration, hypotension, hypovolemia, orthostatic hypotension, and syncope) were reported by 0.3%, 0.5%, and 0.3% of patients treated with placebo, JARDIANCE 10 mg, and JARDIANCE 25 mg respectively. JARDIANCE may increase the risk of hypotension in patients at risk for volume contraction.
Increased Urination
- In the pool five placebo-controlled clinical trials, adverse reactions of increased urination (e.g., polyuria, pollakiuria, and nocturia) occurred more frequently on JARDIANCE than on placebo (see Table 1). Specifically, nocturia was reported by 0.4%, 0.3%, and 0.8% of patients treated with placebo, JARDIANCE 10 mg, and JARDIANCE 25 mg, respectively.
Impairment in Renal Function
- Use of JARDIANCE was associated with increases in serum creatinine and decreases in eGFR (see Table 2). Patients with moderate renal impairment at baseline had larger mean changes.
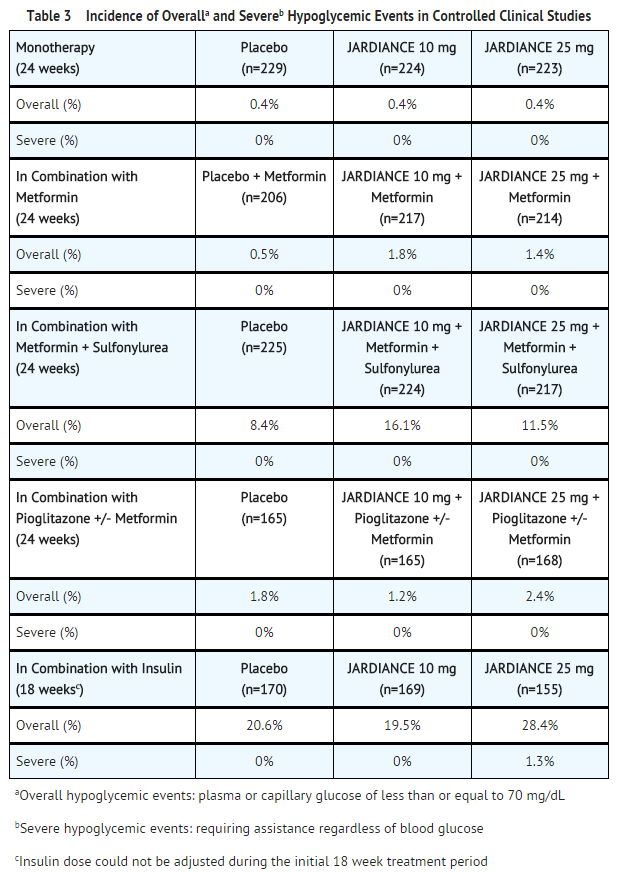
Hypoglycemia
- The incidence of hypoglycemia by study is shown in Table 3. The incidence of hypoglycemia increased when JARDIANCE was administered with insulin or sulfonylurea
Genital Mycotic Infections
- In the pool five placebo-controlled clinical trials, the incidence of genital mycotic infections (e.g., vaginal mycotic infection, vaginal infection, genital infection fungal, vulvovaginal candidiasis, and vulvitis) was increased in patients treated with JARDIANCE compared to placebo, occurring in 0.9%, 4.1%. and 3.7% of patients randomized to placebo, JARDIANCE 10 mg, and JARDIANCE 25 mg, respectively. Discontinuation from study due to genital infection occurred in 0% of placebo-treated patients and 0.2% of patients treated with either JARDIANCE 10 or 25 mg.
- Genital mycotic infections occurred more frequently in female than male patients (see Table 1).
- Phimosis occurred more frequently in male patients treated with JARDIANCE 10 mg (less than 0.1%) and JARDIANCE 25 mg (0.1%) than placebo (0%).
Urinary Tract Infections
- In the pool five placebo-controlled clinical trials, the incidence of urinary tract infections (e.g., urinary tract infection, asymptomatic bacteriuria, and cystitis) was increased in patients treated with JARDIANCE compared to placebo (see Table 1). Patients with a history of chronic or recurrent urinary tract infections were more likely to experience a urinary tract infection. The rate of treatment discontinuation due to urinary tract infections was 0.1%, 0.2%, and 0.1% for placebo, JARDIANCE 10 mg, and JARDIANCE 25 mg, respectively.
- Urinary tract infections occurred more frequently in female patients. The incidence of urinary tract infections in female patients randomized to placebo, JARDIANCE 10 mg, and JARDIANCE 25 mg was 16.6%, 18.4%, and 17.0%, respectively. The incidence of urinary tract infections in male patients randomized to placebo, JARDIANCE 10 mg, and JARDIANCE 25 mg was 3.2%, 3.6%, and 4.1%, respectively .
Laboratory Tests
- Increase in Low-Density Lipoprotein Cholesterol (LDL-C)
- Dose-related increases in low-density lipoprotein cholesterol (LDL-C) were observed in patients treated with JARDIANCE. LDL-C increased by 2.3%, 4.6%, and 6.5% in patients treated with placebo, JARDIANCE 10 mg, and JARDIANCE 25 mg, respectively. The range of mean baseline LDL-C levels was 90.3 to 90.6 mg/dL across treatment groups.
- Increase in Hematocrit
- In a pool of four placebo-controlled studies, median hematocrit decreased by 1.3% in placebo and increased by 2.8% in JARDIANCE 10 mg and 2.8% in JARDIANCE 25 mg treated patients. At the end of treatment, 0.6%, 2.7%, and 3.5% of patients with hematocrits initially within the reference range had values above the upper limit of the reference range with placebo, JARDIANCE 10 mg, and JARDIANCE 25 mg, respectively.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Empagliflozin in the drug label.
Drug Interactions
Diuretics
- Coadministration of empagliflozin with diuretics resulted in increased urine volume and frequency of voids, which might enhance the potential for volume depletion .
Insulin or Insulin Secretagogues
- Coadministration of empagliflozin with insulin or insulin secretagogues increases the risk for hypoglycemia
Positive Urine Glucose Test
- Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors as SGLT2 inhibitors increase urinary glucose excretion and will lead to positive urine glucose tests. Use alternative methods to monitor glycemic control.
Interference with 1,5-anhydroglucitol (1,5-AG) Assay
- Monitoring glycemic control with 1,5-AG assay is not recommended as measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies of JARDIANCE in pregnant women. JARDIANCE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Based on results from animal studies, empagliflozin may affect renal development and maturation. In studies conducted in rats, empagliflozin crosses the placenta and reaches fetal tissues. During pregnancy, consider appropriate alternative therapies, especially during the second and third trimesters.
- Empagliflozin was not teratogenic in embryo-fetal development studies in rats and rabbits up to 300 mg/kg/day, which approximates 48-times and 128-times, respectively, the maximum clinical dose of 25 mg. At higher doses, causing maternal toxicity, malformations of limb bones increased in fetuses at 700 mg/kg/day or 154 times the 25 mg maximum clinical dose in rats. In the rabbit, higher doses of empagliflozin resulted in maternal and fetal toxicity at 700 mg/kg/day, or 139 times the 25 mg maximum clinical dose.
- In pre- and postnatal development studies in pregnant rats, empagliflozin was administered from gestation day 6 through to lactation day 20 (weaning) at up to 100 mg/kg/day (approximately 16 times the 25 mg maximum clinical dose) without maternal toxicity. Reduced body weight was observed in the offspring at greater than or equal to 30 mg/kg/day (approximately 4 times the 25 mg maximum clinical dose).
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Empagliflozin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Empagliflozin during labor and delivery.
Nursing Mothers
It is not known if JARDIANCE is excreted in human milk. Empagliflozin is secreted in the milk of lactating rats reaching levels up to 5 times higher than that in maternal plasma. Since human kidney maturation occurs in utero and during the first 2 years of life when lactational exposure may occur, there may be risk to the developing human kidney. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from JARDIANCE, a decision should be made whether to discontinue nursing or to discontinue JARDIANCE, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of JARDIANCE in pediatric patients under 18 years of age have not been established.
Geriatic Use
- No JARDIANCE dosage change is recommended based on age. A total of 2721 (32%) patients treated with empagliflozin were 65 years of age and older, and 491 (6%) were 75 years of age and older. JARDIANCE is expected to have diminished efficacy in elderly patients with renal impairment [see Use in Specific Populations (8.6)]. The risk of volume depletion-related adverse reactions increased in patients who were 75 years of age and older to 2.1%, 2.3%, and 4.4% for placebo, JARDIANCE 10 mg, and JARDIANCE 25 mg. The risk of urinary tract infections increased in patients who were 75 years of age and older to 10.5%, 15.7%, and 15.1% in patients randomized to placebo, JARDIANCE 10 mg, and JARDIANCE 25 mg, respectively
Gender
There is no FDA guidance on the use of Empagliflozin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Empagliflozin with respect to specific racial populations.
Renal Impairment
- The efficacy and safety of JARDIANCE were evaluated in a study of patients with mild and moderate renal impairment [see Clinical Studies (14.3)]. In this study, 195 patients exposed to JARDIANCE had an eGFR between 60 and 90 mL/min/1.73 m2, 91 patients exposed to JARDIANCE had an eGFR between 45 and 60 mL/min/1.73 m2 and 97 patients exposed to JARDIANCE had an eGFR between 30 and 45 mL/min/1.73 m2. The glucose lowering benefit of JARDIANCE 25 mg decreased in patients with worsening renal function. The risks of renal impairment [see Warnings and Precautions (5.2)], volume depletion adverse reactions and urinary tract infection-related adverse reactions increased with worsening renal function.
- The efficacy and safety of JARDIANCE have not been established in patients with severe renal impairment, with ESRD, or receiving dialysis. JARDIANCE is not expected to be effective in these patient populations
Hepatic Impairment
- JARDIANCE may be used in patients with hepatic impairment
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Empagliflozin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Empagliflozin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Empagliflozin in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Empagliflozin in the drug label.
Overdosage
- In the event of an overdose with JARDIANCE, contact the Poison Control Center. Employ the usual supportive measures (e.g., remove unabsorbed material from the gastrointestinal tract, employ clinical monitoring, and institute supportive treatment) as dictated by the patient’s clinical status. Removal of empagliflozin by hemodialysis has not been studied.
Pharmacology
 | |
| Clinical data | |
|---|---|
| Trade names | Jardiance |
| AHFS/Drugs.com | jardiance |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| ChEBI | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C23H27ClO7 |
| Molar mass | 450.91 g/mol |
| 3D model (JSmol) | |
| |
| |
Mechanism of Action
- Sodium-glucose co-transporter 2 (SGLT2) is the predominant transporter responsible for reabsorption of glucose from the glomerular filtrate back into the circulation. Empagliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, empagliflozin reduces renal reabsorption of filtered glucose and lowers the renal threshold for glucose, and thereby increases urinary glucose excretion.
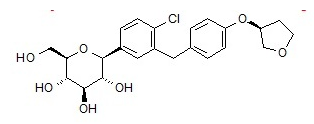
Structure
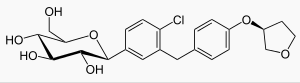
- JARDIANCE tablets contain empagliflozin, an orally-active inhibitor of the sodium-glucose co-transporter 2 (SGLT2).
- The chemical name of empagliflozin is D-Glucitol,1,5-anhydro-1-C-4-chloro-3-4-(3S)-tetrahydro-3-furanyloxyphenylmethylphenyl-,(1S).
- Its molecular formula is C23H27ClO7 and the molecular weight is 450.91. The structural formula is:
Pharmacodynamics
Urinary Glucose Excretion
- In patients with type 2 diabetes, urinary glucose excretion increased immediately following a dose of JARDIANCE and was maintained at the end of a 4-week treatment period averaging at approximately 64 grams per day with 10 mg empagliflozin and 78 grams per day with 25 mg JARDIANCE once daily.
Urinary Volume
- In a 5-day study, mean 24-hour urine volume increase from baseline was 341 mL on Day 1 and 135 mL on Day 5 of empagliflozin 25 mg once daily treatment.
Cardiac Electrophysiology
- In a randomized, placebo-controlled, active-comparator, crossover study, 30 healthy subjects were administered a single oral dose of JARDIANCE 25 mg, JARDIANCE 200 mg (8 times the maximum dose), moxifloxacin, and placebo. No increase in QTc was observed with either 25 mg or 200 mg empagliflozin.
Pharmacokinetics
Absorption
- The pharmacokinetics of empagliflozin has been characterized in healthy volunteers and patients with type 2 diabetes and no clinically relevant differences were noted between the two populations. After oral administration, peak plasma concentrations of empagliflozin were reached at 1.5 hours post-dose. Thereafter, plasma concentrations declined in a biphasic manner with a rapid distribution phase and a relatively slow terminal phase. The steady state mean plasma AUC and Cmax were 1870 nmol·h/L and 259 nmol/L, respectively, with 10 mg empagliflozin once daily treatment, and 4740 nmol·h/L and 687 nmol/L, respectively, with 25 mg empagliflozin once daily treatment. Systemic exposure of empagliflozin increased in a dose-proportional manner in the therapeutic dose range. The single-dose and steady-state pharmacokinetic parameters of empagliflozin were similar, suggesting linear pharmacokinetics with respect to time.
- Administration of 25 mg empagliflozin after intake of a high-fat and high-calorie meal resulted in slightly lower exposure; AUC decreased by approximately 16% and Cmax decreased by approximately 37%, compared to fasted condition. The observed effect of food on empagliflozin pharmacokinetics was not considered clinically relevant and empagliflozin may be administered with or without food.
Distribution
- The apparent steady-state volume of distribution was estimated to be 73.8 L based on a population pharmacokinetic analysis. Following administration of an oral [14C]-empagliflozin solution to healthy subjects, the red blood cell partitioning was approximately 36.8% and plasma protein binding was 86.2%.
Metabolism
- No major metabolites of empagliflozin were detected in human plasma and the most abundant metabolites were three glucuronide conjugates (2-O-, 3-O-, and 6-O-glucuronide). Systemic exposure of each metabolite was less than 10% of total drug-related material. In vitro studies suggested that the primary route of metabolism of empagliflozin in humans is glucuronidation by the uridine 5'-diphospho-glucuronosyltransferases UGT2B7, UGT1A3, UGT1A8, and UGT1A9.
Elimination
- The apparent terminal elimination half-life of empagliflozin was estimated to be 12.4 h and apparent oral clearance was 10.6 L/h based on the population pharmacokinetic analysis. Following once-daily dosing, up to 22% accumulation, with respect to plasma AUC, was observed at steady-state, which was consistent with empagliflozin half-life. Following administration of an oral [14C]-empagliflozin solution to healthy subjects, approximately 95.6% of the drug-related radioactivity was eliminated in feces (41.2%) or urine (54.4%). The majority of drug-related radioactivity recovered in feces was unchanged parent drug and approximately half of drug-related radioactivity excreted in urine was unchanged parent drug.
Specific Populations
Renal Impairment
- In patients with mild (eGFR: 60 to less than 90 mL/min/1.73 m2), moderate (eGFR: 30 to less than 60 mL/min/1.73 m2), and severe (eGFR: less than 30 mL/min/1.73 m2) renal impairment and subjects with kidney failure/end stage renal disease (ESRD) patients, AUC of empagliflozin increased by approximately 18%, 20%, 66%, and 48%, respectively, compared to subjects with normal renal function. Peak plasma levels of empagliflozin were similar in subjects with moderate renal impairment and kidney failure/ESRD compared to patients with normal renal function. Peak plasma levels of empagliflozin were roughly 20% higher in subjects with mild and severe renal impairment as compared to subjects with normal renal function. Population pharmacokinetic analysis showed that the apparent oral clearance of empagliflozin decreased, with a decrease in eGFR leading to an increase in drug exposure. However, the fraction of empagliflozin that was excreted unchanged in urine, and urinary glucose excretion, declined with decrease in eGFR.
Hepatic Impairment
- In subjects with mild, moderate, and severe hepatic impairment according to the Child-Pugh classification, AUC of empagliflozin increased by approximately 23%, 47%, and 75%, and Cmax increased by approximately 4%, 23%, and 48%, respectively, compared to subjects with normal hepatic function.
Effects of Age, Body Mass Index, Gender, and Race
- Based on the population PK analysis, age, body mass index (BMI), gender and race (Asians versus primarily Whites) do not have a clinically meaningful effect on pharmacokinetics of empagliflozin .
Pediatric
- Studies characterizing the pharmacokinetics of empagliflozin in pediatric patients have not been performed.
Drug Interactions
- In vitro Assessment of Drug Interactions
- In vitro data suggest that the primary route of metabolism of empagliflozin in humans is glucuronidation by the uridine 5'-diphospho-glucuronosyltransferases UGT2B7, UGT1A3, UGT1A8, and UGT1A9. Empagliflozin does not inhibit, inactivate, or induce CYP450 isoforms. Empagliflozin also does not inhibit UGT1A1. Therefore, no effect of empagliflozin is anticipated on concomitantly administered drugs that are substrates of the major CYP450 isoforms or UGT1A1. The effect of UGT induction (e.g., induction by rifampicin or any other UGT enzyme inducer) on empagliflozin exposure has not been evaluated.
- Empagliflozin is a substrate for P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), but it does not inhibit these efflux transporters at therapeutic doses. Based on in vitro studies, empagliflozin is considered unlikely to cause interactions with drugs that are P-gp substrates. Empagliflozin is a substrate of the human uptake transporters OAT3, OATP1B1, and OATP1B3, but not OAT1 and OCT2. Empagliflozin does not inhibit any of these human uptake transporters at clinically relevant plasma concentrations and, therefore, no effect of empagliflozin is anticipated on concomitantly administered drugs that are substrates of these uptake transporters.
- In vivo Assessment of Drug Interactions
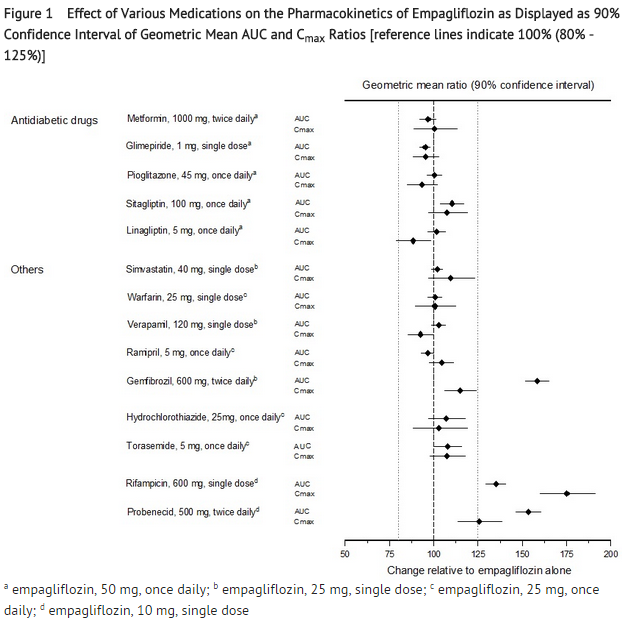
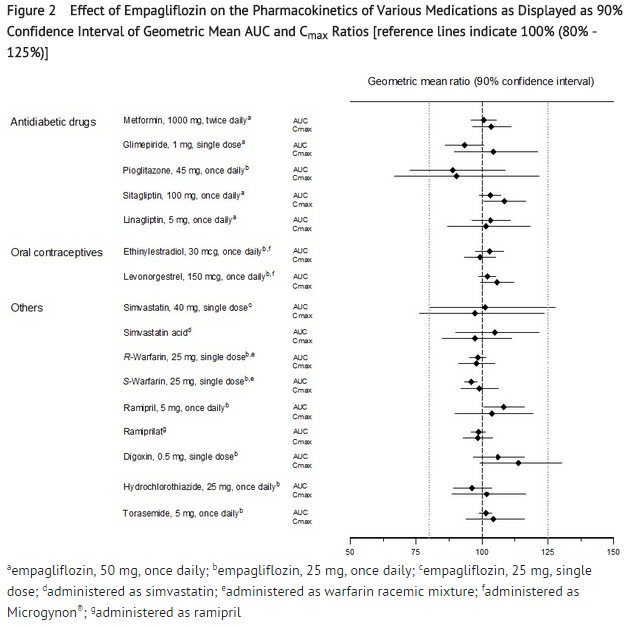
- No dose adjustment of JARDIANCE is recommended when coadministered with commonly prescribed medicinal products based on results of the described pharmacokinetic studies. Empagliflozin pharmacokinetics were similar with and without coadministration of metformin, glimepiride, pioglitazone, sitagliptin, linagliptin, warfarin, verapamil, ramipril, simvastatin, hydrochlorothiazide, and torasemide in healthy volunteers (see Figure 1). The observed increases in overall exposure (AUC) of empagliflozin following coadministration with gemfibrozil, rifampicin, or probenecid are not clinically relevant. In subjects with normal renal function, coadministration of empagliflozin with probenecid resulted in a 30% decrease in the fraction of empagliflozin excreted in urine without any effect on 24-hour urinary glucose excretion. The relevance of this observation to patients with renal impairment is unknown.
- Empagliflozin had no clinically relevant effect on the pharmacokinetics of metformin, glimepiride, pioglitazone, sitagliptin, linagliptin, warfarin, digoxin, ramipril, simvastatin, hydrochlorothiazide, torasemide, and oral contraceptives when coadministered in healthy volunteers (see Figure 2).
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Carcinogenesis was evaluated in 2-year studies conducted in CD-1 mice and Wistar rats. Empagliflozin did not increase the incidence of tumors in female rats dosed at 100, 300, or 700 mg/kg/day (up to 72 times the exposure from the maximum clinical dose of 25 mg). In male rats, hemangiomas of the mesenteric lymph node were increased significantly at 700 mg/kg/day or approximately 42 times the exposure from a 25 mg clinical dose. Empagliflozin did not increase the incidence of tumors in female mice dosed at 100, 300, or 1000 mg/kg/day (up to 62 times the exposure from a 25 mg clinical dose). Renal tubule adenomas and carcinomas were observed in male mice at 1000 mg/kg/day, which is approximately 45 times the exposure of the maximum clinical dose of 25 mg.
Mutagenesis
- Empagliflozin was not mutagenic or clastogenic with or without metabolic activation in the in vitro Ames bacterial mutagenicity assay, the in vitro L5178Y tk+/- mouse lymphoma cell assay, and an in vivo micronucleus assay in rats.
Impairment of Fertility
- Empagliflozin had no effects on mating, fertility or early embryonic development in treated male or female rats up to the high dose of 700 mg/kg/day (approximately 155 times the 25 mg clinical dose in males and females, respectively).
Clinical Studies
- JARDIANCE has been studied as monotherapy and in combination with metformin, sulfonylurea, pioglitazone, and insulin. JARDIANCE has also been studied in patients with type 2 diabetes with mild or moderate renal impairment.
- In patients with type 2 diabetes, treatment with JARDIANCE reduced hemoglobin A1c (HbA1c), compared to placebo. The reduction in HbA1c for JARDIANCE compared with placebo was observed across subgroups including gender, race, geographic region, baseline BMI and duration of disease.
Monotherapy
- A total of 986 patients with type 2 diabetes participated in a double-blind, placebo-controlled study to evaluate the efficacy and safety of JARDIANCE monotherapy.
- Treatment-naïve patients with inadequately controlled type 2 diabetes entered an open-label placebo run-in for 2 weeks. At the end of the run-in period, patients who remained inadequately controlled and had an HbA1c between 7 and 10% were randomized to placebo, JARDIANCE 10 mg, JARDIANCE 25 mg, or a reference comparator.
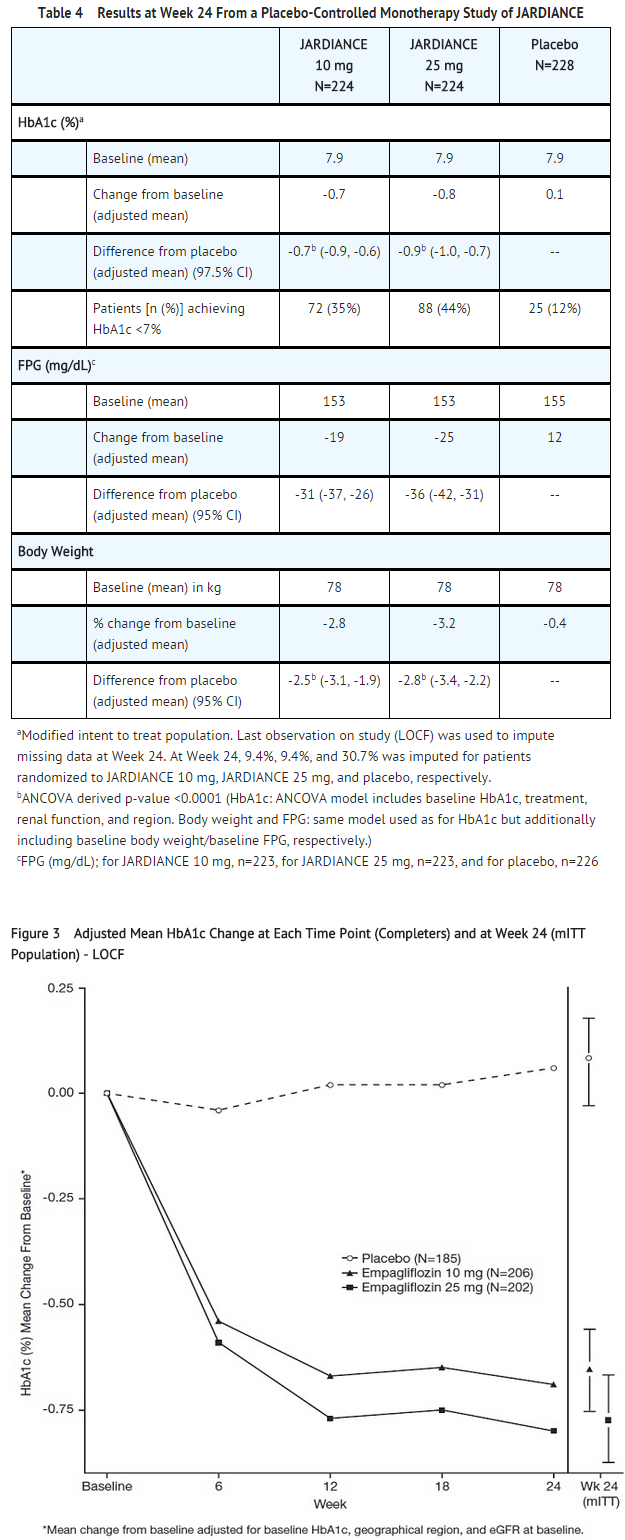
- At Week 24, treatment with JARDIANCE 10 mg or 25 mg daily provided statistically significant reductions in HbA1c (p-value <0.0001), fasting plasma glucose (FPG), and body weight compared with placebo (see Table 4 and Figure 3).
- At Week 24, the systolic blood pressure was statistically significantly reduced compared to placebo by -2.6 mmHg (placebo-adjusted, p-value=0.0231) in patients randomized to 10 mg of JARDIANCE and by -3.4 mmHg (placebo-corrected, p-value=0.0028) in patients randomized to 25 mg of JARDIANCE.
Combination Therapy
- Add-On Combination Therapy with Metformin
- A total of 637 patients with type 2 diabetes participated in a double-blind, placebo-controlled study to evaluate the efficacy and safety of JARDIANCE in combination with metformin.
- Patients with type 2 diabetes inadequately controlled on at least 1500 mg of metformin per day entered an open-label 2 week placebo run-in. At the end of the run-in period, patients who remained inadequately controlled and had an HbA1c between 7 and 10% were randomized to placebo, JARDIANCE 10 mg, or JARDIANCE 25 mg.
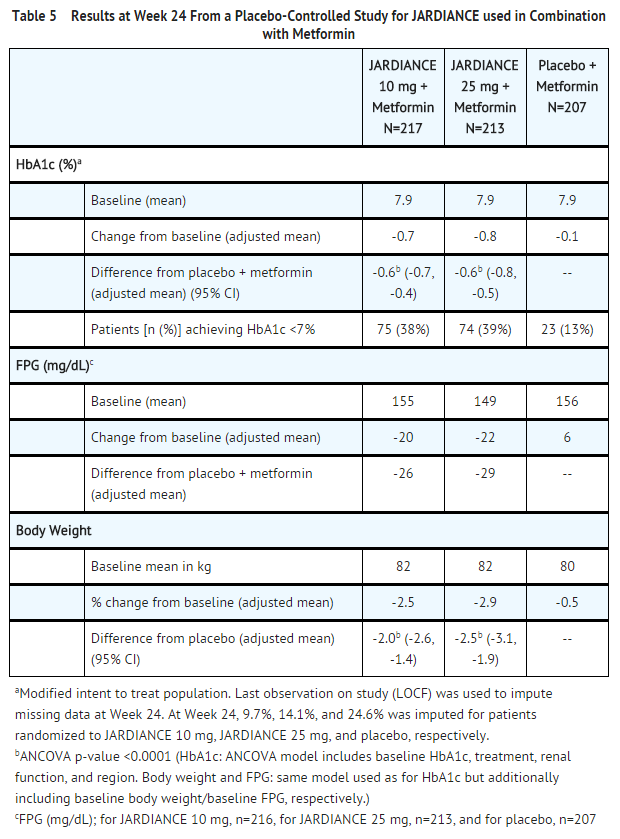
- At Week 24, treatment with JARDIANCE 10 mg or 25 mg daily provided statistically significant reductions in HbA1c (p-value <0.0001), FPG, and body weight compared with placebo (see Table 5).
- At Week 24, the systolic blood pressure was statistically significantly reduced compared to placebo by -4.1 mmHg (placebo-corrected, p-value <0.0001) for JARDIANCE 10 mg and -4.8 mmHg (placebo-corrected, p-value <0.0001) for JARDIANCE 25 mg.
- Add-On Combination Therapy with Metformin and Sulfonylurea
- A total of 666 patients with type 2 diabetes participated in a double-blind, placebo-controlled study to evaluate the efficacy and safety of JARDIANCE in combination with metformin plus a sulfonylurea.
- Patients with inadequately controlled type 2 diabetes on at least 1500 mg per day of metformin and on a sulfonylurea, entered a 2 week open-label placebo run-in. At the end of the run-in, patients who remained inadequately controlled and had an HbA1c between 7% and 10% were randomized to placebo, JARDIANCE 10 mg, or JARDIANCE 25 mg.
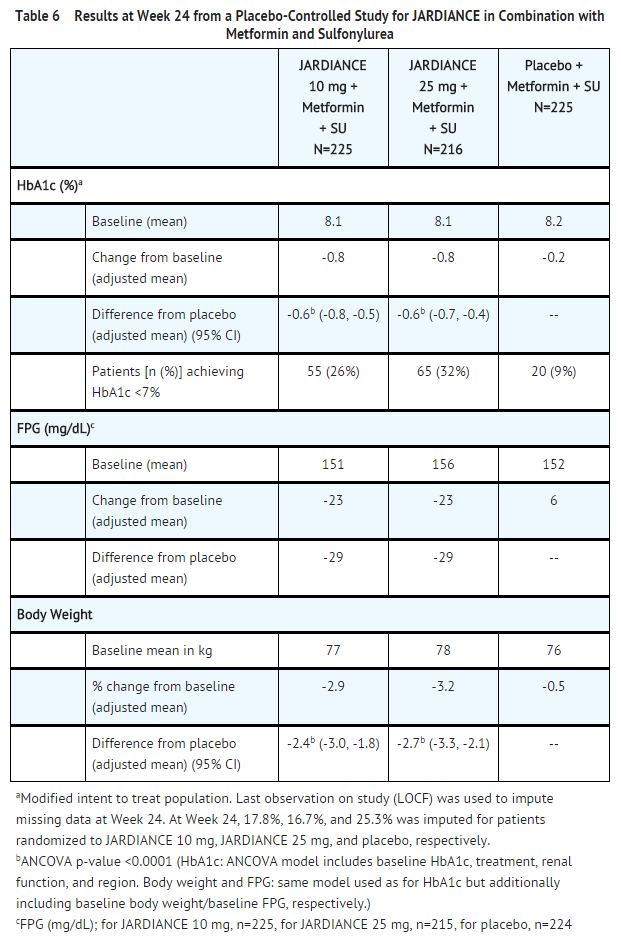
- Treatment with JARDIANCE 10 mg or 25 mg daily provided statistically significant reductions in HbA1c (p-value <0.0001), FPG, and body weight compared with placebo (see Table 6).
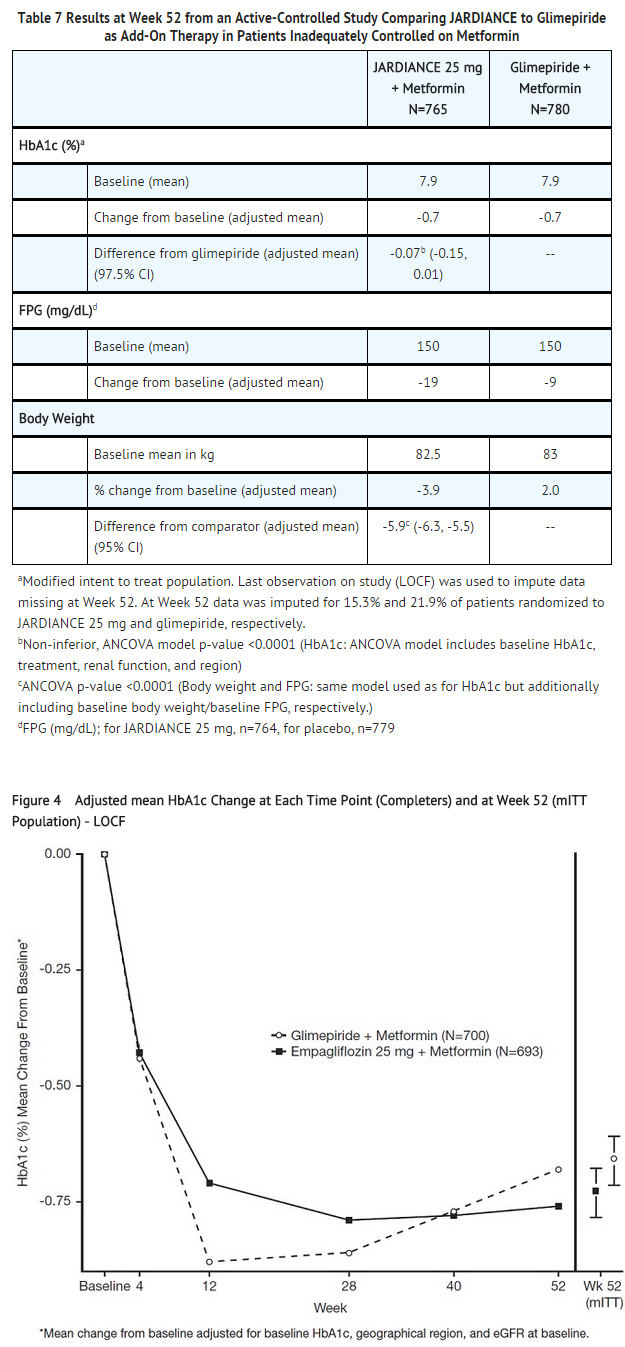
- Active-Controlled Study versus Glimepiride in Combination with Metformin
- The efficacy of JARDIANCE was evaluated in a double-blind, glimepiride-controlled, study in 1545 patients with type 2 diabetes with insufficient glycemic control despite metformin therapy.
- Patients with inadequate glycemic control and an HbA1c between 7% and 10% after a 2-week run-in period were randomized to glimepiride or JARDIANCE 25 mg.
- At Week 52, JARDIANCE 25 mg and glimepiride lowered HbA1c and FPG (see Table 7, Figure 4). The difference in observed effect size between JARDIANCE 25 mg and glimepiride excluded the pre-specified non-inferiority margin of 0.3%. The mean daily dose of glimepiride was 2.7 mg and the maximal approved dose in the United States is 8 mg per day.
- At Week 52, the adjusted mean change from baseline in systolic blood pressure was -3.6 mmHg, compared to 2.2 mmHg for glimepiride. The differences between treatment groups for systolic blood pressure was statistically significant (p-value <0.0001).
- Add-On Combination Therapy with Pioglitazone with or without Metformin
- A total of 498 patients with type 2 diabetes participated in a double-blind, placebo-controlled study to evaluate the efficacy and safety of JARDIANCE in combination with pioglitazone, with or without metformin.
- Patients with inadequately controlled type 2 diabetes on metformin at a dose of at least 1500 mg per day and pioglitazone at a dose of at least 30 mg per day were placed into an open-label placebo run-in for 2 weeks. Patients with inadequate glycemic control and an HbA1c between 7% and 10% after the run-in period were randomized to placebo, JARDIANCE 10 mg, or JARDIANCE 25 mg.
- Treatment with JARDIANCE 10 mg or 25 mg daily resulted in statistically significant reductions in HbA1c (p-value <0.0001), FPG, and body weight compared with placebo (see Table 8).
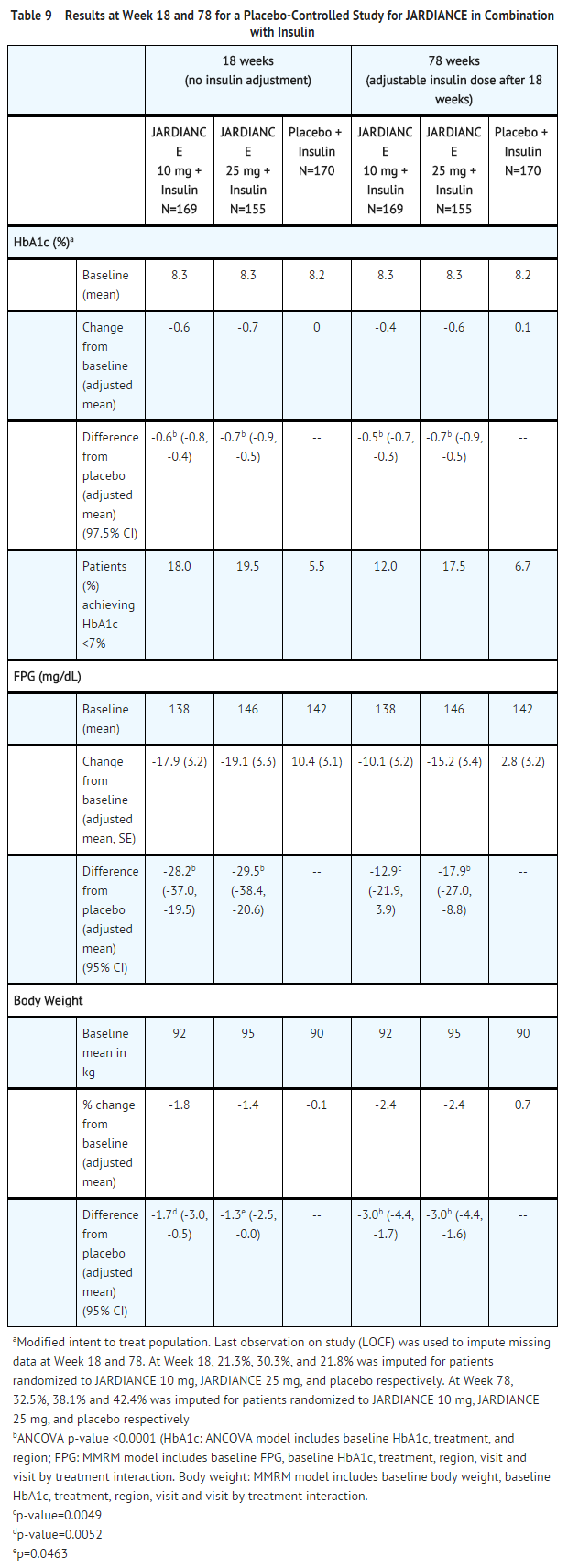
- Add-On Combination with Insulin with or without Metformin and/or Sulfonylureas
- A total of 494 patients with type 2 diabetes inadequately controlled on insulin, or insulin in combination with oral drugs participated in a double-blind, placebo-controlled study to evaluate the efficacy of JARDIANCE as add-on therapy to insulin over 78 weeks.
- Patients entered a 2-week placebo run-in period on basal insulin (e.g., insulin glargine, insulin detemir, or NPH insulin) with or without metformin and/or sulfonylurea background therapy. Following the run-in period, patients with inadequate glycemic control were randomized to the addition of JARDIANCE 10 mg, JARDIANCE 25 mg, or placebo. Patients were maintained on a stable dose of insulin prior to enrollment, during the run-in period, and during the first 18 weeks of treatment. For the remaining 60 weeks, insulin could be adjusted. The mean total daily insulin dose at baseline for JARDIANCE 10 mg, 25 mg, and placebo was 45 IU, 48 IU, and 48 IU, respectively.
- JARDIANCE used in combination with insulin (with or without metformin and/or sulfonylurea) provided statistically significant reductions in HbA1c and FPG compared to placebo after both 18 and 78 weeks of treatment (see Table 9). JARDIANCE 10 mg or 25 mg daily also resulted in statistically significantly greater percent body weight reduction compared to placebo.
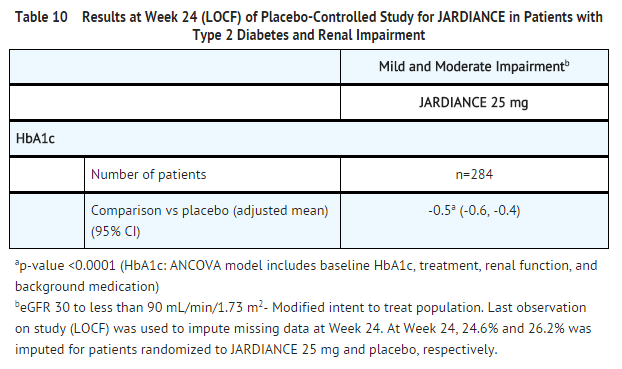
Renal Impairment
- A total of 738 patients with type 2 diabetes and a baseline eGFR less than 90 mL/min/1.73 m2 participated in a randomized, double-blind, placebo-controlled, parallel-group to evaluate the efficacy and safety of JARDIANCE in patients with type 2 diabetes and renal impairment. The trial population comprised of 290 patients with mild renal impairment (eGFR 60 to less than 90 mL/min/1.73 m2), 374 patients with moderate renal impairment (eGFR 30 to less than 60 mL/min/1.73 m2), and 74 with severe renal impairment (eGFR less than 30 mL/min/1.73 m2). A total of 194 patients with moderate renal impairment had a baseline eGFR of 30 to less than 45 mL/min/1.73 m2 and 180 patients a baseline eGFR of 45 to less than 60 mL/min/1.73 m2.
- At Week 24, JARDIANCE 25 mg provided statistically significant reduction in HbA1c relative to placebo in patients with mild to moderate renal impairment (see Table 10). A statistically significant reduction relative to placebo was also observed with JARDIANCE 25 mg in patients with either mild [-0.7 (95% CI: -0.9, -0.5)] or moderate [-0.4 (95% CI: -0.6, -0.3)] renal impairment and with JARDIANCE 10 mg in patients with mild [-0.5 (95% CI: -0.7, -0.3)] renal impairment.
- The glucose lowering efficacy of JARDIANCE 25 mg decreased with decreasing level of renal function in the mild to moderate range. Least square mean Hb1Ac changes at 24 weeks were -0.6%, -0.5%, and -0.2% for those with a baseline eGFR of 60 to less than 90 mL/min/1.73 m2, 45 to less than 60 mL/min/1.73 m2, and 30 to less than 45 mL/min/1.73 m2, respectively [see Dosage and Administration (2) and Use in Specific Populations (8.6)]. For placebo, least square mean HbA1c changes at 24 weeks were 0.1%, -0.1%, and 0.2% for patients with a baseline eGFR of 60 to less than 90 mL/min/1.73 m2, 45 to less than 60 mL/min/1.73 m2, and 30 to less than 45 mL/min/1.73 m2, respectively.
- For patients with severe renal impairment, the analyses of changes in HbA1c and FPG showed no discernible treatment effect of JARDIANCE 25 mg compared to placebo
How Supplied
- JARDIANCE tablets are available in 10 mg and 25 mg strengths as follows:
10 mg tablets: pale yellow, round, biconvex and bevel-edged, film-coated tablets debossed with “S 10” on one side and the Boehringer Ingelheim company symbol on the other side. Bottles of 30 (NDC 0597-0152-30) Bottles of 90 (NDC 0597-0152-90) Cartons containing 3 blister cards of 10 tablets each (3 x 10) (NDC 0597-0152-37), institutional pack.
25 mg tablets: pale yellow, oval, biconvex film-coated tablets, debossed with “S 25” on one side and the Boehringer Ingelheim company symbol on the other side. Bottles of 30 (NDC 0597-0153-30) Bottles of 90 (NDC 0597-0153-90) Cartons containing 3 blister cards of 10 tablets each (3 x 10) (NDC 0597-0153-37), institutional pack.
Dispense in a well-closed container as defined in the USP.
Storage
- Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F)
Images
Drug Images
{{#ask: Page Name::Empagliflozin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PATIENT PACKAGE INSERT
PATIENT INFORMATION
JARDIANCE® (jar DEE ans) (empagliflozin) Tablets
Read this Patient Information before you start taking JARDIANCE and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or treatment.
What is the most important information I should know about JARDIANCE? JARDIANCE can cause serious side effects, including:
Dehydration. JARDIANCE can cause some people to have dehydration (the loss of body water and salt). Dehydration may cause you to feel dizzy, faint, light-headed, or weak, especially when you stand up (orthostatic hypotension).
You may be at higher risk of dehydration if you: have low blood pressure take medicines to lower your blood pressure, including diuretics (water pill) are on low sodium (salt) diet have kidney problems are 65 years of age or older Vaginal yeast infection. Women who take JARDIANCE may get vaginal yeast infections. Symptoms of a vaginal yeast infection include: vaginal odor white or yellowish vaginal discharge (discharge may be lumpy or look like cottage cheese) vaginal itching Yeast infection of the penis (balanitis or balanoposthitis). Men who take JARDIANCE may get a yeast infection of the skin around the penis. Certain men who are not circumcised may have swelling of the penis that makes it difficult to pull back the skin around the tip of the penis. Other symptoms of yeast infection of the penis include: redness, itching, or swelling of the penis rash of the penis foul smelling discharge from the penis pain in the skin around penis Talk to your doctor about what to do if you get symptoms of a yeast infection of the vagina or penis. Your doctor may suggest you use an over-the-counter antifungal medicine. Talk to your doctor right away if you use an over-the-counter antifungal medication and your symptoms do not go away.
What is JARDIANCE?
JARDIANCE is a prescription medicine used along with diet and exercise to lower blood sugar in adults with type 2 diabetes. JARDIANCE is not for people with type 1 diabetes. JARDIANCE is not for people with diabetic ketoacidosis (increased ketones in the blood or urine). It is not known if JARDIANCE is safe and effective in children under 18 years of age. Who should not take JARDIANCE?
Do not take JARDIANCE if you:
are allergic to empagliflozin or any of the ingredients in JARDIANCE. See the end of this leaflet for a list of ingredients in JARDIANCE. have severe kidney problems or are on dialysis What should I tell my doctor before using JARDIANCE?
Before you take JARDIANCE, tell your doctor if you:
have kidney problems have liver problems have a history of urinary tract infections or problems with urination have any other medical conditions are pregnant or planning to become pregnant. It is not known if JARDIANCE will harm your unborn baby. If you are pregnant, talk with your doctor about the best way to control your blood sugar while you are pregnant. are breastfeeding or plan to breastfeed. It is not known if JARDIANCE passes into your breast milk. Talk with your doctor about the best way to feed your baby if you take JARDIANCE. Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
JARDIANCE may affect the way other medicines work, and other medicines may affect how JARDIANCE works.
Especially tell your doctor if you take:
diuretics (water pills) insulin or other medicines that can lower your blood sugar Ask your doctor or pharmacist for a list of these medicines if you are not sure if your medicine is listed above.
How should I take JARDIANCE?
Take JARDIANCE exactly as your doctor tells you to take it. Take JARDIANCE by mouth 1 time in the morning each day, with or without food. Your doctor may change your dose if needed. If you miss a dose, take it as soon as you remember. If you do not remember until it is time for your next dose, skip the missed dose and go back to your regular schedule. Do not take two doses of JARDIANCE at the same time. Talk with your doctor if you have questions about a missed dose. Your doctor may tell you to take JARDIANCE along with other diabetes medicines. Low blood sugar can happen more often when JARDIANCE is taken with certain other diabetes medicines. See “What are the possible side effects of JARDIANCE?” If you take too much JARDIANCE, call your doctor or go to the nearest hospital emergency room right away. When your body is under some types of stress, such as fever, trauma (such as a car accident), infection, or surgery, the amount of diabetes medicine that you need may change. Tell your doctor right away if you have any of these conditions and follow your doctor’s instructions. Check your blood sugar as your doctor tells you to. Stay on your prescribed diet and exercise program while taking JARDIANCE. Talk to your doctor about how to prevent, recognize and manage low blood sugar (hypoglycemia), high blood sugar (hyperglycemia), and complications of diabetes. Your doctor will check your diabetes with regular blood tests, including your blood sugar levels and your hemoglobin HbA1c. When taking JARDIANCE, you may have sugar in your urine, which will show up on a urine test. What are the possible side effects of JARDIANCE?
JARDIANCE may cause serious side effects, including:
See “What is the most important information I should know about JARDIANCE?” low blood sugar (hypoglycemia). If you take JARDIANCE with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin, your risk of getting low blood sugar is higher. The dose of your sulfonylurea medicine or insulin may need to be lowered while you take JARDIANCE. Signs and symptoms of low blood sugar may include: headache irritability drowsiness hunger weakness fast heart beat dizziness sweating confusion shaking or feeling jittery
kidney problems, especially in people 75 years of age or older and people who already have kidney problems increased fats in your blood (cholesterol) The most common side effects of JARDIANCE include:
urinary tract infection. Signs and symptoms of a urinary tract infection may include burning feeling when passing urine, urine that looks cloudy, pain in the pelvis, or back pain. These are not all the possible side effects of JARDIANCE. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store JARDIANCE?
Store JARDIANCE at room temperature between 68°F to 77°F (20°C to 25°C).
General information about the safe and effective use of JARDIANCE.
This Patient Information summarizes the most important information about JARDIANCE. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about JARDIANCE that is written for health professionals.
PRINCIPAL DISPLAY PANEL
JARDIANCE 10 mg - Carton NDC: 0597-0152-37
PRINCIPAL DISPLAY PANEL
JARDIANCE 10 mg - Bottle NDC: 0597-0152-30
PRINCIPAL DISPLAY PANEL
JARDIANCE 25 mg - Carton NDC: 0597-0153-37
PRINCIPAL DISPLAY PANEL
JARDIANCE 25 mg - Bottle NDC: 0597-0153-30
Ingredients and Appearance
{{#ask: Label Page::Empagliflozin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Patient Information).
Instructions
- Instruct patients to read the Patient Information before starting JARDIANCE therapy and to reread it each time the prescription is renewed. Instruct patients to inform their doctor or pharmacist if they develop any unusual symptom, or if any known symptom persists or worsens.
- Inform patients of the potential risks and benefits of JARDIANCE and of alternative modes of therapy. Also inform patients about the importance of adherence to dietary instructions, regular physical activity, periodic blood glucose monitoring and HbA1c testing, recognition and management of hypoglycemia and hyperglycemia, and assessment for diabetes complications. Advise patients to seek medical advice promptly during periods of stress such as fever, trauma, infection, or surgery, as medication requirements may change.
- Instruct patients to take JARDIANCE only as prescribed. If a dose is missed, it should be taken as soon as the patient remembers. Advise patients not to double their next dose.
- Inform patients that the most common adverse reactions associated with the use of JARDIANCE are urinary tract infections and mycotic genital infections.
- Inform female patients of child bearing age that the use of JARDIANCE during pregnancy has not been studied in humans, and that JARDIANCE should only be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Based on animal data, JARDIANCE may cause fetal harm in the second and third trimesters. Instruct patients to report pregnancies to their physicians as soon as possible.
- Inform nursing mothers to discontinue JARDIANCE or nursing, taking into account the important of the drug to the mother. It is not known if JARDIANCE is excreted in breast milk; however, based on animal data, JARDIANCE may cause harm to nursing infants.
Hypotension
- Inform patients that hypotension may occur with JARDIANCE and advise them to contact their healthcare provider if they experience such symptoms. Inform patients that dehydration may increase the risk for hypotension, and to have adequate fluid intake.
Urinary Tract Infections
- Inform patients of the potential for urinary tract infections. Provide them with information on the symptoms of urinary tract infections. Advise them to seek medical advice if such symptoms occur.
Genital Mycotic Infections in Females (e.g., Vulvovaginitis)
- Inform female patients that vaginal yeast infections may occur and provide them with information on the signs and symptoms of vaginal yeast infections. Advise them of treatment options and when to seek medical advice .
Genital Mycotic Infections in Males (e.g., Balanitis or Balanoposthitis)
- Inform male patients that yeast infection of penis (e.g., balanitis or balanoposthitis) may occur, especially in uncircumcised males and patients with chronic and recurrent infections. Provide them with information on the signs and symptoms of balanitis and balanoposthitis (rash or redness of the glans or foreskin of the penis). Advise them of treatment options and when to seek medical advice.
Laboratory Tests
- Inform patients that renal function should be assessed prior to initiation of JARDIANCE and monitored periodically thereafter.
- Inform patients that elevated glucose in urinalysis is expected when taking JARDIANCE.
- Inform patients that response to all diabetic therapies should be monitored by periodic measurements of blood glucose and HbA1c levels, with a goal of decreasing these levels toward the normal range. Hemoglobin A1c monitoring is especially useful for evaluating long-term glycemic control.
Precautions with Alcohol
- Alcohol-Empagliflozin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- JARDIANCE®[1]
Look-Alike Drug Names
There is limited information regarding Empagliflozin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Empagliflozin
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Empagliflozin |Label Name=
}}
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles with non-default infobox title
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles containing unverified chemical infoboxes
- Drug